Ready-to-Use Premix Medications.
A broad portfolio of premixes that are there when your patients need them.
Baxter Ready-to-Use (RTU)
Baxter offers practical and innovative products in premix ready-to-use IV formulations, which may help increase hospital efficiency, while helping with your overall safety protocols.1,2
Ready for Safety:
• Consistent drug concentrations may help minimize medication errors associated with compounding1,2
• Barcoded for bedside scanning to help ensure the right patient gets the right medication1
Ready for Efficiency:
• Improved workflow by allowing on-demand availability of medication, which may help reduce the steps required to prepare medication for administration
• Supports inventory management and helps reduce waste3
Ready for Value:
• Manufacturer-prepared premix medications have a shelf life of 9 to 24 months
• Proprietary GALAXY container technology—a non-PVC and non-DEHP system—enables certain premix medicines to have an extended shelf life when stored at room temperature
Ready for Patient Care:
• Designed for ready use, helping to reduce the risk of preventable medication errors in patients associated with compounding1,2
• Formulations that are shelf-stable at room temperature allow for storage in automated dispensing cabinets and closer to the patient
Baxter’s manufacturer-prepared premixes are convenient and ready when you need them, potentially shortening the time between ordering and administration. This may allow you to spend more time with your patients.
Now… Learn More About our Premixes
See Our Value to Customers



Baxter's Partnership Promise
For over 90 years, Baxter has been focused on helping support patient care.
We are committed to partnering with healthcare facilities to help solve their most pressing challenges, including supporting patient safety, operational efficiencies and worker shortages.

Featured Ready-to-Use Products
Baxter offers a breadth of ready-to-use medications, both liquid and frozen, to help your healthcare facility meet the needs of your patients and helping to ensure they receive optimal care from the ER to the OR and beyond.
Please see accompanying Indications, Important Risk Information, and link to the Prescribing
Information for each of these products below.
Ready to purchase? Call your Baxter sales representative, or dial: 1.800.229.0001
Passive Thawing—Frozen Premix Medications Video
Thawing frozen premix medications is an important step in preparing a hospital for a full day of patient care. And trying to minimize the number of touchpoints in the process is key to creating efficiencies. Watch the Passive Medication-Thawing Video to learn more about how this may be a solution to your thawing needs.
Premix Manufacturing Process Video
Baxter has developed a premix manufacturing process in the US specifically designed for long-term storage of unstable and temperature-sensitive medications. This may help reduce waste,3 streamline workflow, support efficient delivery of medications, and reduce human errors related to admixing.1,2
Watch the Manufacturing Video to learn about our cGMP-compliant manufacturing process.
Find additional product information in our online catalog or contact us to learn more about our broad portfolio of premix ready-to-use medications.
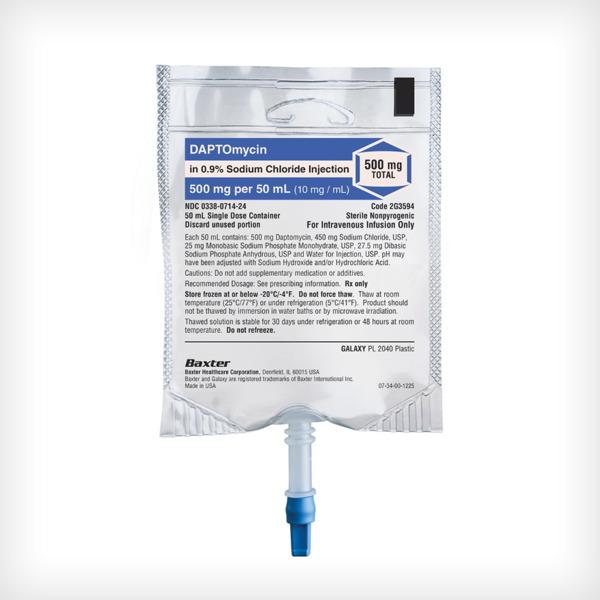
Daptomycin in 0.9% Sodium Chloride Injection
350 mg/50 mL, 500 mg/50 mL, 700 mg/100 mL, 1,000 mg/100 mL
Indications and Important Risk Information
Indications
Daptomycin in Sodium Chloride Injection is a lipopeptide antibacterial indicated for the treatment of:
• Complicated skin and skin structure infections (cSSSI) in adult and pediatric patients (1 to 17 years of age) for whom appropriate dosing can be achieved and,
• Staphylococcus aureus bloodstream infections (bacteremia), in adult patients for whom appropriate dosing can be achieved, including those with right-sided infective endocarditis,
• Staphylococcus aureus bloodstream infections (bacteremia) in pediatric patients (1 to 17 years of age) for whom appropriate dosing can be achieved.
Limitations of Use:
• Daptomycin in Sodium Chloride Injection is not indicated for the treatment of pneumonia.
• Daptomycin in Sodium Chloride Injection is not indicated for the treatment of left-sided infective endocarditis due to S. aureus.
• Daptomycin in Sodium Chloride Injection is not recommended in pediatric patients younger than one year of age due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Daptomycin in Sodium Chloride Injection and other antibacterial drugs, Daptomycin in Sodium Chloride Injection should be used to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Important Risk Information
Contraindications
• Daptomycin in Sodium Chloride Injection is contraindicated in patients with a known hypersensitivity to daptomycin.
Warnings and Precautions
• Anaphylaxis/Hypersensitivity Reactions: Anaphylaxis/hypersensitivity reactions have been reported with the use of antibacterial agents, including daptomycin for injection, and may be life-threatening. If an allergic reaction occurs, discontinue the drug and institute appropriate therapy.
• Myopathy and Rhabdomyolysis: Patients receiving Daptomycin in Sodium Chloride Injection should be monitored for the development of muscle pain or weakness, particularly of the distal extremities. CPK levels should be monitored weekly, and more frequently in patients who received recent, prior, or concomitant therapy with an HMG-CoA reductase inhibitor or in whom elevations in CPK occur during treatment. In adult patients with renal impairment, both renal function and CPK should be monitored more frequently than once weekly.
Daptomycin in Sodium Chloride Injection should not be dosed more frequently than once a day.
Daptomycin in Sodium Chloride Injection should be discontinued in patients with unexplained signs and symptoms of myopathy in conjunction with CPK elevations to levels >1,000 U/L, and in patients without reported symptoms who have marked elevations in CPK, with levels >2,000 U/L.
• Eosinophilic Pneumonia: Has been reported in patients receiving daptomycin for injection. In reported cases, patients developed fever, dyspnea with hypoxic respiratory insufficiency, and diffuse pulmonary infiltrates or organizing pneumonia. In general, patients developed eosinophilic pneumonia 2 to 4 weeks after starting daptomycin for injection and improved when discontinued and steroid therapy was initiated. Recurrence of eosinophilic pneumonia upon re-exposure has been reported. Patients who develop these signs and symptoms should undergo prompt medical evaluation, and Daptomycin in Sodium Chloride Injection should be discontinued immediately.
• Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): DRESS has been reported in postmarketing experience. Patients who develop skin rash, fever, peripheral eosinophilia, and systemic organ (for example, hepatic, renal, pulmonary) impairment while receiving Daptomycin in Sodium Chloride Injection should undergo medical evaluation. If DRESS is suspected, discontinue promptly and institute appropriate treatment.
• Tubulointerstitial Nephritis (TIN): TIN has been reported in post-marketing experience. Patients who develop new or worsening renal impairment while receiving Daptomycin in Sodium Chloride Injection should undergo medical evaluation. If TIN is suspected, discontinue promptly and institute appropriate treatment.
• Peripheral Neuropathy: Cases have been reported during post-marketing experience. Therefore, physicians should be alert to signs and symptoms of peripheral neuropathy in patients receiving Daptomycin in Sodium Chloride Injection. Monitor for neuropathy and consider discontinuation.
• Potential Nervous System and/or Muscular System Effects in Pediatric Patients Younger than 12 Months: Avoid use in pediatric patients younger than 12 months due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs with intravenous daptomycin.
• Clostridioides difficile-Associated Diarrhea (CDAD): CDAD has been reported with the use of nearly all systemic antibacterial agents, including daptomycin for injection, and may range in severity from mild diarrhea to fatal colitis. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
• Persisting or Relapsing S. aureus Bacteremia/Endocarditis: Patients should have repeat blood cultures. If a blood culture is positive for S. aureus, minimum inhibitory concentration (MIC) susceptibility testing of the isolate should be performed, and diagnostic evaluation of the patient should be performed to rule out sequestered foci of infection. Appropriate surgical intervention and/or consideration of a change in antibacterial regimen may be required. Failure of treatment may be due to reduced daptomycin susceptibility.
• Decreased efficacy was observed in adult patients with moderate baseline renal impairment: Consider these data when selecting antibacterial therapy for use in adult patients with baseline moderate to severe renal impairment.
• Adverse Reactions:
- Adult cSSSI Patients: The most common adverse reactions that occurred in ≥2% of adult cSSSI patients receiving daptomycin for injection 4 mg/kg were diarrhea, headache, dizziness, rash, abnormal liver function tests, elevated creatine phosphokinase (CPK), urinary tract infections, hypotension, and dyspnea.
- Pediatric cSSSI Patients: The most common adverse reactions that occurred in ≥2% of pediatric patients receiving daptomycin for injection were diarrhea, vomiting, abdominal pain, pruritus, pyrexia, elevated CPK, and headache.
- Adult S. aureus bacteremia/endocarditis Patients: The most common adverse reactions that occurred in ≥5% of S. aureus bacteremia/endocarditis patients receiving daptomycin for injection 6 mg/kg were sepsis, bacteremia, abdominal pain, chest pain, edema, pharyngolaryngeal pain, pruritus, increased sweating, insomnia, elevated CPK, and hypertension.
- Pediatric S. aureus bacteremia Patients: The most common adverse reactions that occurred in ≥5% of pediatric patients receiving daptomycin for injection were vomiting and elevated CPK.
• Drug Interactions:
- HMG-CoA Reductase Inhibitors: Inhibitors of HMG-CoA reductase may cause myopathy. Experience with the coadministration of HMG-CoA reductase inhibitors and daptomycin for injection in patients is limited; therefore, consideration should be given to suspending use of HMG-CoA reductase inhibitors temporarily in patients receiving Daptomycin in Sodium Chloride Injection.
- Drug-Lab Test Interactions: Increased International Normalized Ratio (INR)/Prolonged Prothrombin Time: Clinically relevant plasma concentrations of daptomycin have been observed to cause a significant concentration-dependent false prolongation of prothrombin time (PT) and elevation of International Normalized Ratio (INR) when certain recombinant thromboplastin reagents are utilized for the assay.
Dosage and Administration
• If a dose of Daptomycin in Sodium Chloride Injection is required that does not equal 350 mg, 500 mg, 700 mg or 1,000 mg, this product is not recommended for use and an alternative formulation of daptomycin should be considered.
Please see accompanying full Prescribing Information for Daptomycin in 0.9% Sodium Chloride Injection.
ZOSYN (piperacillin and tazobactam) Injection
2.25 g/50 mL, 3.375 g/50 mL, 4.5 g/ 100 mL
Indications and Important Risk Information
Indications
Zosyn is a combination of piperacillin, a penicillin-class antibacterial and tazobactam, a beta-lactamase inhibitor, indicated for the treatment of:
• Intra-abdominal infections in adult and pediatric patients 2 months of age and older
• Nosocomial pneumonia in adult and pediatric patients 2 months of age and older
• Skin and skin structure infections in adults
• Female pelvic infections in adults
• Community-acquired pneumonia in adults
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Zosyn and other antibacterial drugs, Zosyn should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
Contraindications
Zosyn is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or beta-lactamase inhibitors.
Warnings and Precautions
• Hypersensitivity Adverse Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with Zosyn. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. If an allergic reaction occurs, Zosyn should be discontinued and appropriate therapy instituted.
• Severe Cutaneous Adverse Reactions: Zosyn may cause severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis. If patients develop a skin rash they should be monitored closely and Zosyn discontinued if lesions progress.
• Hemophagocytic Lymphohistiocytosis (HLH): Cases of HLH have been reported in pediatric and adult patients treated with Zosyn. Signs and symptoms of HLH may include fever, rash, lymphadenopathy, hepatosplenomegaly and cytopenia. If HLH is suspected, discontinue Zosyn immediately and institute appropriate management.
• Hematologic Adverse Reactions: Bleeding manifestations have occurred in some patients receiving beta-lactam drugs, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, Zosyn should be discontinued and appropriate therapy instituted. The leukopenia/neutropenia associated with Zosyn administration appears to be reversible and most frequently associated with prolonged administration.
Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy, i.e., ≥ 21 days.
• Central Nervous System Adverse Reactions: As with other penicillins, Zosyn may cause neuromuscular excitability or seizures. Patients receiving higher doses, especially patients with renal impairment may be at greater risk for central nervous system adverse reactions. Closely monitor patients with renal impairment or seizure disorders for signs and symptoms of neuromuscular excitability or seizures.
• Nephrotoxicity in Critically Ill Patients: The use of Zosyn was found to be an independent risk factor for renal failure and was associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs in critically ill patients. Alternative treatment options should be considered in the critically ill population. If alternative treatment options are inadequate or unavailable, monitor renal function during treatment with Zosyn.
• Clostridioides difficile-associated diarrhea (CDAD): CDAD has been reported with use of nearly all antibacterial agents, including Zosyn, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
• Adverse Reactions: The most common adverse reactions (incidence >5%) are diarrhea, constipation, nausea, headache, and insomnia.
• Renal Impairment: In patients with creatinine clearance ≤ 40 mL/min and dialysis patients (hemodialysis and CAPD), the intravenous dose of Zosyn should be reduced to the degree of renal function impairment.
• Drug Interactions:
- Zosyn administration can significantly reduce tobramycin concentrations in hemodialysis patients. Monitor tobramycin concentrations in these patients.
- Probenecid prolongs the half-lives of piperacillin and tazobactam and should not be co-administered with Zosyn unless the benefit outweighs the risk.
- Co-administration of Zosyn with vancomycin may increase the incidence of acute kidney injury. Monitor kidney function in patients receiving Zosyn and vancomycin.
- Monitor coagulation parameters in patients receiving Zosyn and heparin or oral anticoagulants.
- Zosyn may prolong the neuromuscular blockade of vecuronium and other non-depolarizing neuromuscular blockers. Monitor for adverse reactions related to neuromuscular blockade.
Please see accompanying full Prescribing Information for Zosyn.
Norepinephrine Bitartrate in 5% Dextrose Injection
4 mg equivalent of norepinephrine (16 mcg/mL) in 5% dextrose 250 mL
8 mg equivalent of norepinephrine (32 mcg/mL) in 5% dextrose 250 mL
16 mg equivalent of norepinephrine (64 mcg/mL) in 5% dextrose 250 mL
Indication and Important Risk Information
Indication
• Norepinephrine Bitartrate in Dextrose Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
Important Risk Information
• Contraindications: None.
• Tissue Ischemia: Administration of Norepinephrine Bitartrate in Dextrose Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction, decreased renal perfusion and reduced urine output, tissue hypoxia, lactic acidosis, and reduced systemic blood flow despite “normal” blood pressure. Address hypovolemia prior to initiating Norepinephrine Bitartrate in Dextrose Injection. Avoid use in patients with mesenteric or peripheral vascular thrombosis, as this may increase ischemia and extend the area of infarction.
Gangrene of the extremities has occurred in patients with occlusive or thrombotic vascular disease or who received prolonged or high dose infusions. Monitor for changes to the skin of the extremities in susceptible patients.
Extravasation of Norepinephrine Bitartrate in Dextrose Injection may cause necrosis and sloughing of surrounding tissue. To reduce the risk of extravasation, infuse into a large vein, check the infusion site frequently for free flow, and monitor for signs of extravasation.
Avoid administration into the veins in the leg in elderly patients.
Emergency Treatment of Extravasation: Infiltrate the ischemic area as soon as possible, using a syringe with a fine hypodermic needle with 5 to 10 mg of phentolamine mesylate in 10 to 15 mL of 0.9% Sodium Chloride Injection in adults.
• Hypotension after Abrupt Discontinuation: Sudden cessation of the infusion rate may result in marked hypotension. When discontinuing the infusion, gradually reduce the infusion rate while expanding blood volume with intravenous fluids.
• Cardiac Arrhythmias: Norepinephrine Bitartrate in Dextrose Injection elevates intracellular calcium concentrations and may cause arrhythmias, particularly in the setting of hypoxia or hypercarbia. Perform continuous cardiac monitoring of patients with arrhythmias.
• Elderly Patients: May be at a greater risk of developing adverse reactions.
• Adverse Reactions: Most common adverse reactions are hypertension and bradycardia.
• Drug Interactions:
- Co-administration of Norepinephrine Bitartrate in Dextrose Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g., linezolid) or with tricyclic antidepressants can cause severe, prolonged hypertension.
- Anti-diabetics: Norepinephrine Bitartrate in Dextrose Injection can decrease insulin sensitivity and raise blood glucose.
- Concomitant use of Norepinephrine Bitartrate in Dextrose Injection with halogenated anesthetics may lead to ventricular tachycardia or ventricular fibrillation. Monitor cardiac rhythm in patients receiving concomitant halogenated anesthetics.
Please see accompanying full Prescribing Information for Norepinephrine Bitartrate in 5% Dextrose Injection.
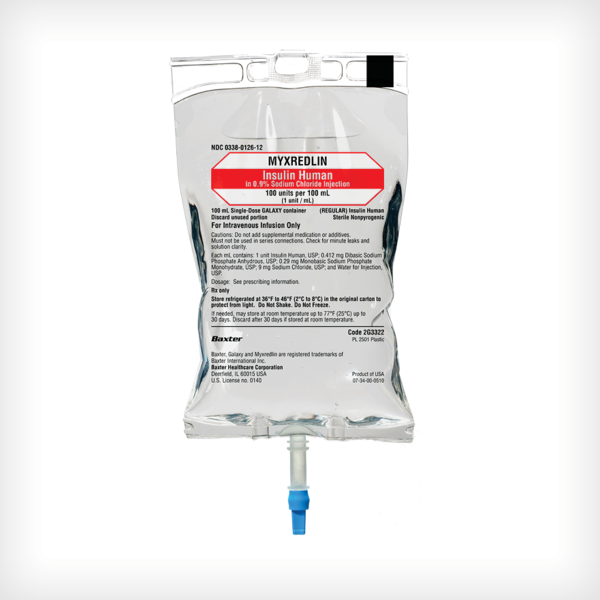
MYXREDLIN (Insulin Human) in 0.9% Sodium Chloride Injection
100 units/100 mL (1 unit/mL)
INDICATIONS AND IMPORTANT RISK INFORMATION
Indication
MYXREDLIN is a short-acting human insulin indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
Important Risk Information
Contraindications
• During episodes of hypoglycemia
• Hypersensitivity to insulin human or any of the excipients in MYXREDLIN
Warnings and Precautions
• Hyper- or Hypoglycemia with Changes in Insulin Regimen: Carry out under close medical supervision and increase frequency of blood glucose monitoring.
• Administer MYXREDLIN intravenously ONLY under medical supervision with close monitoring of blood glucose and potassium levels. Hypokalemia may be life-threatening if not treated.
• Individualize dose based on metabolic needs, blood glucose monitoring results, and glycemic control goal. Dosage adjustments may be needed with changes in nutrition, renal, or hepatic function or during acute illness.
• Adverse reactions observed with insulin human injection include hypoglycemia, allergic reactions, weight gain and edema.
• Fluid Retention and Heart Failure with Concomitant Use of Thiazolidinediones (TZDs): Observe for signs and symptoms of heart failure; such as shortness of breath, swelling of your ankles or feet, or sudden weight gain.
Dosage and Administration
• Inspect MYXREDLIN visually before use. It should appear clear and colorless. Do not use MYXREDLIN if particulate matter or coloration is seen.
• Do not add supplementary medication or additives.
• Do not use in series connections.
• Do not shake or freeze. Discard unused portion.
Please see accompanying full Prescribing Information for MYXREDLIN.
NEXTERONE (amiodarone HCl) Premixed Injection
150 mg/100 mL and 360 mg/200 mL
Indication and Important Risk Information
Indication
NEXTERONE is indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients refractory to other therapy.
Important Risk Information
Contraindications
NEXTERONE is contraindicated in patients with:
• Known hypersensitivity to any of the components of NEXTERONE, including iodine
• Cardiogenic shock
• Marked sinus bradycardia
• Second- or third-degree atrioventricular (AV) block unless a functioning pacemaker is available
Warnings and Precautions
• Persistence of Adverse Effects: Because of the long half-life of amiodarone (9 to 36 days) and its metabolite desethylamiodarone (9 to 30 days), adverse reactions or interactions, as well as observed adverse effects, can persist following amiodarone withdrawal.
• Hypotension: Most often seen in the first several hours of treatment and likely related to the rate of infusion. In some cases, hypotension may be refractory and result in a fatal outcome.
To treat: Slow the infusion; as needed, add vasopressor drugs, positive inotropic agents, and volume expansion.
• Bradycardia and Atrioventricular Block: May require slowing the infusion rate or discontinuing NEXTERONE. In some patients, inserting a pacemaker is required. Have a temporary pacemaker available when treating a patient predisposed to bradycardia or AV block.
• Hepatic Injury: Acute hepatocellular necrosis leading to hepatic coma, acute renal failure, and death has been associated. Intravenous infusions at much higher concentrations and rates of infusion than those recommended appear to increase this risk. Carefully monitor patients receiving NEXTERONE for evidence of progressive hepatic injury. Consider reducing the rate of administration or withdrawing NEXTERONE if hepatic injury occurs.
• Proarrhythmia: NEXTERONE may cause a worsening of existing arrhythmias or precipitate a new arrhythmia, sometimes leading to fatal outcomes. Proarrhythmia, primarily torsade de pointes (TdP), has been associated with prolongation by intravenous amiodarone. Monitor patients for QTc prolongation during infusion with NEXTERONE. Reserve the combination of amiodarone with other antiarrhythmic therapies that prolong the QTc to patients with life-threatening ventricular arrhythmias who are incompletely responsive to a single agent. Correct hypokalemia, hypomagnesemia or hypocalcemia whenever possible before initiating treatment with NEXTERONE.
• Pulmonary Injury: There have been post-marketing reports of acute-onset (days to weeks) pulmonary injury. Some cases have progressed to respiratory failure or death. Monitor for new respiratory symptoms and evaluate appropriately. Obtain a baseline chest X-ray and pulmonary function tests in patients who are expected to be receiving amiodarone chronically.
• Loss of Vision: Cases of optic neuropathy and optic neuritis, usually resulting in visual impairment, have been reported. In some cases, visual impairment has progressed to permanent blindness. Optic neuropathy and neuritis may occur at any time following initiation of therapy. Perform an ophthalmic examination if symptoms of visual impairment appear. Reevaluate the necessity of amiodarone therapy if optic neuropathy or neuritis is suspected.
• Thyroid Abnormalities: NEXTERONE inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and may cause increased T3 levels, and increased levels of inactive reverse T3 (rT3) in clinically euthyroid patients. Monitor thyroid function prior to treatment and periodically thereafter, particularly in elderly patients, and in any patient with a history of thyroid nodules, goiter, or other thyroid dysfunction. Hyperthyroidism may induce arrhythmia breakthrough. If any new signs of arrhythmia appear, the possibility of hyperthyroidism should be considered.
• Neonatal Injury: Amiodarone can cause fetal harm when administered to a pregnant woman. Fetal exposure may increase the potential for adverse experiences including cardiac, thyroid, neurodevelopmental, neurological and growth effects in neonate. Inform the patient of the potential hazard to the fetus if NEXTERONE is administered during pregnancy or if the patient becomes pregnant while taking NEXTERONE.
• Hypersensitivity Reactions: Anaphylactic/anaphylactoid reactions have been reported including shock (sometimes fatal), cardiac arrest, and the following manifestations: hypotension, tachycardia, hypoxia, cyanosis, rash, Stevens-Johnson syndrome, flushing, hyperhidrosis and cold sweat.
• Adverse Reactions: The most common adverse reactions (1-2%) leading to discontinuation of intravenous amiodarone therapy are hypotension, asystole/cardiac arrest/pulseless electrical activity, VT, and cardiogenic shock. Other important adverse reactions are torsade de pointes, congestive heart failure, and liver function test abnormalities.
• Drug Interactions: Amiodarone is a substrate for CYP3A and CYP2C8, so inhibitors and inducers affect amiodarone exposure. Amiodarone inhibits p-glycoprotein and CYP1A2, CYP2C9, CYP2D6, and CYP3A, increasing exposure to other drugs.
Please see accompanying full Prescribing Information for NEXTERONE (amiodarone HCl) Premixed Injection.
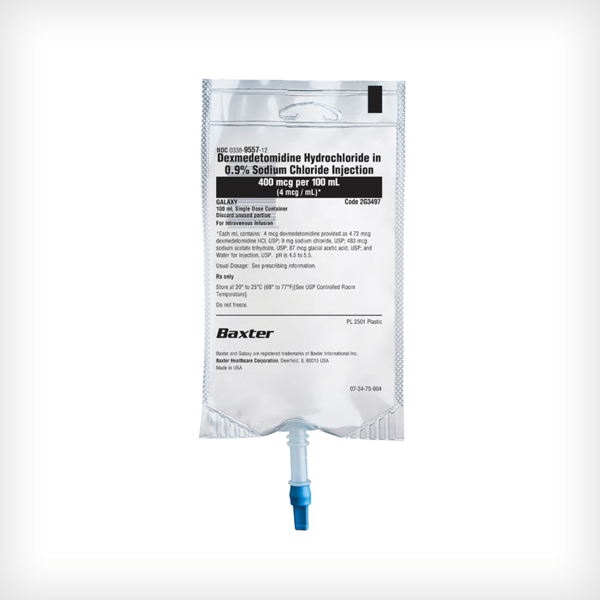
Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection
200 mcg/50 mL (4 mcg/mL) in a 50 mL Galaxy container
400 mcg/100 mL (4 mcg/mL) in a 100 mL Galaxy container
Indications and Important Risk Information
Indications
Dexmedetomidine hydrochloride in 0.9% sodium chloride injection is an alpha2-adrenergic receptor agonist indicated for:
• Sedation of initially intubated and mechanically ventilated adult patients during treatment in an intensive care setting. Administer dexmedetomidine hydrochloride in 0.9% sodium chloride injection by continuous infusion not to exceed 24 hours.
• Sedation of non-intubated adult patients prior to and/or during surgical and other procedures.
Important Risk Information
• Contraindications: None
• Monitoring: Dexmedetomidine hydrochloride in 0.9% sodium chloride injection should be administered only by persons skilled in the management of patients in the intensive care or operating room setting. Patients should be continuously monitored.
• Hypotension, Bradycardia, and Sinus Arrest: Clinically significant episodes of bradycardia and sinus arrest have been reported with administration in young, healthy adult volunteers with high vagal tone or with different routes of administration including rapid intravenous or bolus administration.
Reports of hypotension and bradycardia have been associated with dexmedetomidine hydrochloride in 0.9% sodium chloride injection infusion. Some of these cases have resulted in fatalities. If medical intervention is required, treatment may include decreasing or stopping the infusion, increasing the rate of intravenous fluid administration, elevation of the lower extremities, and use of pressor agents.
Caution should be exercised when administering to patients with advanced heart block and/or severe ventricular dysfunction. Hypotension and/or bradycardia may be expected to be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension and in elderly patients.
Use with caution with co-administration with other vasodilators or negative chronotropic agents.
• Transient Hypertension: Has been observed primarily during the loading dose. Treatment has generally not been necessary, although reduction of the loading infusion rate may be desirable.
• Arousability: Some patients have been observed to be arousable and alert when stimulated. This alone should not be considered as evidence of lack of efficacy in the absence of other clinical signs and symptoms.
• Tolerance and Tachyphylaxis: Use of dexmedetomidine beyond 24 hours has been associated with tolerance and tachyphylaxis and a dose-related increase in adverse reactions.
• Hyperthermia or Pyrexia: May be resistant to traditional cooling methods, such as administration of cooled intravenous fluids and antipyretic medications. Discontinue if drug-related hyperthermia or pyrexia is suspected and monitor patients until body temperature normalizes.
• Hepatic Impairment: Clearance decreases with severity of hepatic impairment, dose reduction should be considered in patients with impaired hepatic function.
• Adverse Reactions:
• The most common adverse reactions (incidence >2%) in adults are hypotension, bradycardia, and dry mouth.
• Adverse reactions in adults, associated with infusions >24 hours in duration include ARDS, respiratory failure, and agitation.
• Drug Interactions:
• Anesthetics, Sedatives, Hypnotics, Opioids: Enhancement of pharmacodynamic effects. Reduction in dosage of dexmedetomidine hydrochloride in 0.9% sodium chloride injection or the concomitant medication may be required.
Please see accompanying full Prescribing Information for Dexmedetomidine Hydrochloride in 0.9% Sodium Chloride Injection.
Vancomycin Injection, USP
Vancomycin Injection, USP in 5% Dextrose
500 mg/100 mL (5 mg/mL) in single dose GALAXY container
750 mg/150 mL (5 mg/mL) in single dose GALAXY container
1 g/200 mL (5 mg/mL) in single dose GALAXY container
1.25 g/250 mL (5 mg/mL) in single dose GALAXY container
1.5 g/300 mL (5 mg/mL) in single dose GALAXY container
Vancomycin Injection, USP in 0.9% Sodium Chloride
500 mg/100 mL (5 mg/mL) in single dose GALAXY container
750 mg/150 mL (5 mg/mL) in single dose GALAXY container
1 g/200 mL (5 mg/mL) in single dose GALAXY container
Indications and Important Risk Information
Indications
Vancomycin is indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant (beta-lactam-resistant) staphylococci. It is indicated for penicillin-allergic patients, for patients who cannot receive or who have failed to respond to other drugs, and for infections caused by vancomycin-susceptible organisms that are resistant to other antimicrobial drugs.
Vancomycin is effective in the treatment of:
- Infective Endocarditis
- staphylococcal endocarditis
- endocarditis caused by Streptococcus viridans or S. bovis, alone or in combination with an aminoglycoside
- endocarditis caused by enterococci (e.g., E. faecalis), only in combination with an aminoglycoside
- diphtheroid endocarditis
- early-onset prosthetic valve endocarditis caused by S. epidermidis or diphtheroids in combination with either rifampin, an aminoglycoside, or both - Septicemia
- Skin and Skin Structure Infection
- Bone Infections
- Lower Respiratory Tract Infections
To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin and other antibacterial drugs, vancomycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
- Contraindications: Vancomycin is contraindicated in patients with known hypersensitivity to this antibiotic. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
- Infusion Reactions: Rapid bolus administration (e.g., over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest.
During or soon after rapid infusion of vancomycin, patients may develop anaphylactoid reactions, including hypotension, wheezing, dyspnea, urticaria, or pruritus. Rapid infusion may also cause flushing of the upper body (“vancomycin infusion reaction”) or pain and muscle spasm of the chest and back.
Vancomycin should be administered over a period of not less than 60 minutes. Stopping the infusion usually results in prompt cessation of these reactions. - Nephrotoxicity: Systemic vancomycin exposure may result in acute kidney injury (AKI). The risk of AKI increases as systemic exposure/serum levels increase. Monitor renal function in all patients; especially with underlying renal impairment, with co-morbidities, and receiving concomitant therapy with a known nephrotoxic drug.
- Ototoxicity: It may be transient or permanent. It has been reported mostly in patients who have been given excessive doses, who have an underlying hearing loss, or who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside. Vancomycin should be used with caution in patients with renal insufficiency.
- Severe Dermatologic Reactions: Toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) have been reported. Cutaneous signs or symptoms reported include skin rashes, mucosal lesions, and blisters. Discontinue Vancomycin Injection at the first appearance of signs and symptoms of TEN, SJS, DRESS, AGEP, or LABD. Dosage of vancomycin must be adjusted for patients with renal dysfunction.
- Clostridioides difficile associated diarrhea (CDAD): May range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
- Hemorrhagic Occlusive Retinal Vasculitis: Including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or the intravitreal route have not been established.
- Adverse Reactions: Not already mentioned above, patients have been reported to have neutropenia, phlebitis, drug fever, nausea, chills, and vasculitis in association with administration of vancomycin.
- Drug Interactions:
- Anesthetic Agents: Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing and anaphylactoid reactions.
- Monitor renal function in patients receiving vancomycin and concurrent and/or sequential systemic or topical use of other potentially neurotoxic and/or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymyxin B, colistin, viomycin, or cisplatin.
Please see accompanying full Prescribing Information for Vancomycin Injection, USP.
Vasopressin in 0.9% Sodium Chloride Injection
20 units vasopressin (0.2 units/mL) in 100 mL 0.9% sodium chloride
40 units vasopressin (0.4 units/mL) in 100 mL 0.9% sodium chloride
Indications and Important Risk Information
Indications
- Vasopressin in Sodium Chloride Injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
Important Risk Information
- Contraindications: Vasopressin in Sodium Chloride Injection is contraindicated in patients with a known allergy or hypersensitivity to 8-L-arginine vasopressin.
- Worsening Cardiac Function: A decrease in cardiac index may be observed with the use of vasopressin.
- Reversible Diabetes Insipidus: Patients may experience reversible diabetes insipidus, manifested by the development of polyuria, a dilute urine, and hypernatremia, after cessation of treatment with vasopressin. Monitor serum electrolytes, fluid status and urine output after vasopressin discontinuation. Some patients may require readministration of vasopressin or administration of desmopressin to correct fluid and electrolyte shifts.
- Adverse Reactions:
- The most common adverse reactions include decreased cardiac output, bradycardia, tachyarrhythmias, hyponatremia and ischemia (coronary, mesenteric, skin, digital).
- Drug Interactions:
- Pressor effects of catecholamines and Vasopressin in Sodium Chloride Injection are expected to be additive.
- Indomethacin may prolong effects of Vasopressin in Sodium Chloride Injection.
- Co-administration of ganglionic blockers or drugs causing SIADH (syndrome of inappropriate antidiuretic hormone secretion) may increase the pressor response.
- Co-administration of drugs causing diabetes insipidus may decrease the pressor response.
- Pregnancy: May induce tonic uterine contractions that could threaten the continuation of pregnancy.
Please see accompanying full Prescribing Information for Vasopressin in 0.9% Sodium Chloride Injection.
Billstein-Leber M, Carrillo JD, Cassano AT, Moline K, Robertson JJ. ASHP guidelines on preventing medication errors in hospitals. Am J Health Syst Pharm. 2018;75(19):1493-1517.
Institute for Safe Medication Practices. ISMP Guidelines for Sterile Compounding and the Safe Use of Sterile Compounding Technology; 2022. https://www.ismp.org/resources/guidelines-sterile-compounding-and-safe-use-sterile-compounding-technology. Updated May 4, 2022. Accessed June 1, 2023.
ASHP Expert Panel on Medication Cost Management. ASHP guidelines on medication cost management strategies for hospitals and health systems.
Am J Health Syst Pharm. 2008;65(14):1368-1384.
Institute for Safe Medication Practices (ISMP). Guidelines for Safe Preparation of Compounded Sterile Preparations; 2016.
Lahue BJ, Pyenson B, Iwasaki K, Blumen HE, Forray S, Rothschild JM. National burden of preventable adverse drug events associated with inpatient injectable medications: healthcare and medical professional liability costs. Am Health Drug Benefits. 2012;5(7):1-10.
Do you want to leave our website?
By continuing, you will leave our website and go to a third-party site. We are not responsible for any content on the third-party site.