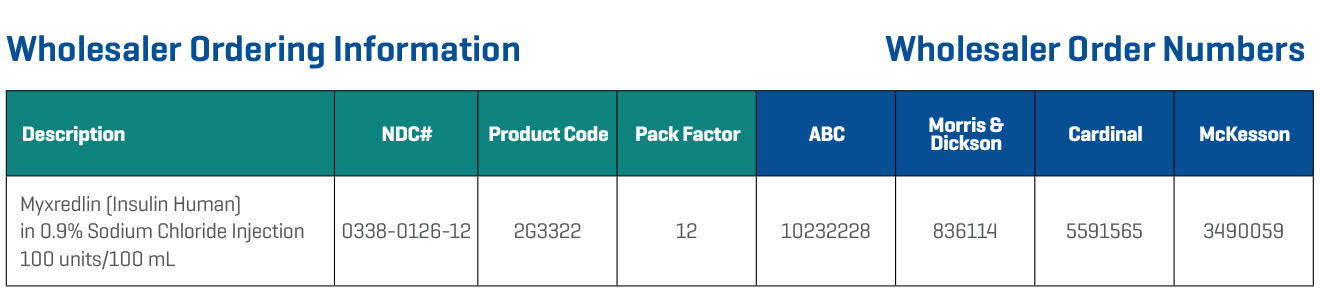
Is Your IV Insulin Ready When and Where You Need It?
Indication
MYXREDLIN is a short-acting human insulin indicated to improve glycemic control in adults and pediatric patients with diabetes mellitus.
Please see the accompanying full Indication(s) and Important Risk Information and full Prescribing Information.
Available when and where you need it: A ready-to-use IV infusion helps reduce patient wait time for high-alert insulin. Myxredlin is ready to scan and hang.
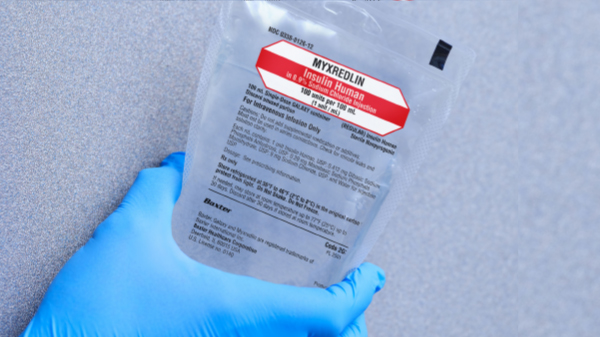
MYXREDLIN helps support safety with:
• Opportunity to reduce medication errors associated with compounding1,2
• Consistent, reliable concentration of a commercially prepared insulin infusion
• On-bag barcoding to help verify patient-medication association
30-Day Room Temperature Storage
• Store closer to the patient
• Help reduce pharmacy STAT production
• If needed, MYXREDLIN may be removed from the original carton and stored at room temperature up to 77°F (25°C) for up to 30 days
24-Month Refrigerated Shelf Life
• Help ensure IV insulin is available when needed, reducing the need for on-the-floor compounding practices
• Store MYXREDLIN in the refrigerator (36°F to 46°F [2°C to 8°C]) in the original carton to protect from light
In a review of preventable adverse drug events (ADE) associated with inpatient injectable medications,* insulin had the highest probability for a preventable adverse drug event3
*Route of administration not specified.
IV insulin ranked #1 for high-alert drugs among pharmacists and nurses4
ISMP and ASHP guidelines recommend use of a commercially prepared product instead of compounding as a risk-reduction strategy for high-alert medications.1,2
When implemented alongside existing hospital best practices, Myxredlin can help reduce errors that may result from compounding practices.1,2
It really only takes one bad outcome associated with compounding to change your mind about using a premix IV product like Myxredlin. But why would you wait for a compounding error? …Every facility doesn’t always have 24-hour pharmacy, or sometimes there’s a STAT order for insulin that needs to be filled. I do not like the idea of asking nursing to compound on the floor, especially during an emergency. Myxredlin lets us keep IV insulin in the automated dispensing cabinet.”
CHRISTUS Health, Tina Collins, PharmD, Director of Corporate Clinical Medication Safety
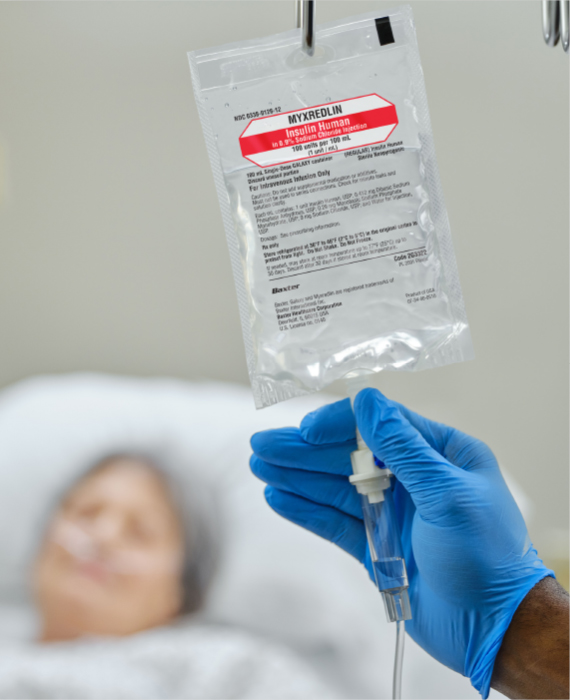
Managing Efficiency with MYXREDLIN
Myxredlin can help
• Streamline insulin production and delivery
• Reduce expired or unused insulin waste5
- No additional costs related to disposal of preservative-free Myxredlin compared with insulin vials containing m-cresol
• Reduce risk of preventable adverse drug events associated with compounding errors1,2 and pharmacy resources spent compounding