WHEN STAT ISN’T FAST ENOUGH
Indication
Norepinephrine Bitartrate in Dextrose Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
Select Important Risk Information
- Tissue Ischemia: Administration of Norepinephrine Bitartrate in Dextrose Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction, decreased renal perfusion and reduced urine output, tissue hypoxia, lactic acidosis, and reduced systemic blood flow despite “normal” blood pressure. Address hypovolemia prior to initiating Norepinephrine Bitartrate in Dextrose Injection. Avoid use in patients with mesenteric or peripheral vascular thrombosis, as this may increase ischemia and extend the area of infarction.
Gangrene of the extremities has occurred in patients with occlusive or thrombotic vascular disease or who received prolonged or high dose infusions. Monitor for changes to the skin of the extremities in susceptible patients.
Please read the accompanying full Indication and Important Risk Information and full Prescribing Information

A Premix from Baxter:
Norepinephrine Bitartrate in 5% Dextrose Injection
The first and only FDA-approved, ready-to-use Premix Norepinephrine in Dextrose
Consistent concentration to help minimize risk of medication errors due to compounding1,2
Reduced wait time for drug administration as compared to compounding
Room temperature storage, making it available near the point of care
See detailed storage information below.
Distinctly labeled, barcoded product bag, with an overwrap that differentiates product strength (concentration)
Patient Safety
Reduction of Medication Errors
Premix Norepinephrine provides an accurately prepared concentration in a barcoded bag to help minimize risk of compounding errors that may be heightened in urgent situations. 1,2
Compliance
Premix Norepinephrine delivers the safety and reliability of compliance with ISMP and ASHP guidelines in a shelf-stable, ready-to-use formulation.1,2
Distinct Labeling
Premix Norepinephrine has a distinctly labeled bag, with a light-shielding overwrap that also differentiates product strength (concentration).
Care and Efficiency
Grab-and-go
Make every second count with a ready-to-use IV medication that helps protect your patients while improving efficiency.
Store Closer to the Patient
Premix Norepinephrine allows for the storage of norepinephrine on the floor and in your ADC, right where you need it when you need it, with reduced concern for waste.3
Operational Efficiency
Premix Norepinephrine may free up your pharmacy staff to focus on compounding other critical high-alert medications.
On-Demand Availability
12 month room temperature shelf life puts Premix Norepinephrine at the point of care.
See detailed storage information below.

Premix Norepinephrine labels include bold colors on the light-shielding overwrap and barcodes to help reduce the potential of medication errors.2
Order Premix Norepinephrine Now
Light-shielding
-
One side of the bag has amber film
-
One side of the bag has aluminum foil
Storage
In overwrap, room temperature 20°C to 25°C (68°F to 77°F)*: 12 month shelf-life or until manufacturer expiration date, whichever is earlier
Out of overwrap, room temperature 20°C to 25°C (68°F to 77°F)*: 30 days stability or until manufacturer expiration date, whichever is earlier
* Excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
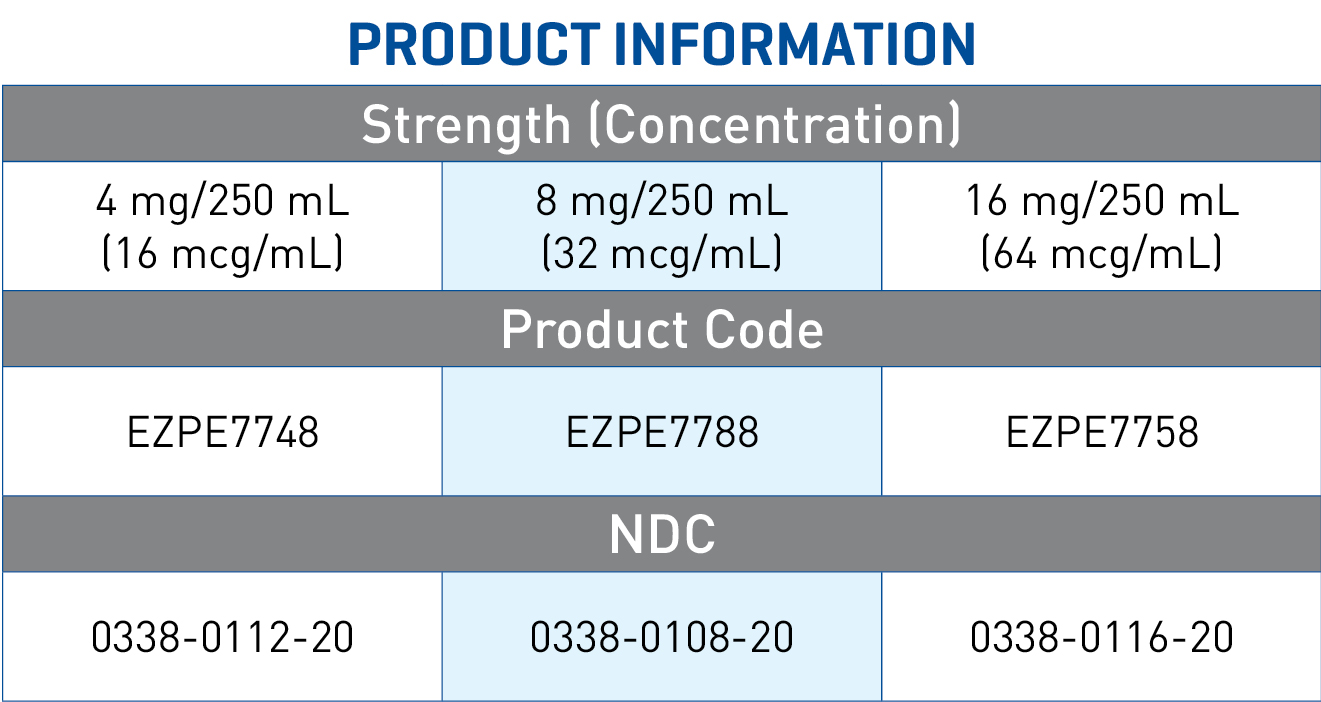
Norepinephrine Bitartrate in 5% Dextrose Injection
4 mg equivalent of norepinephrine (16 mcg/mL) in 5% dextrose 250 mL
8 mg equivalent of norepinephrine (32 mcg/mL) in 5% dextrose 250 mL
16 mg equivalent of norepinephrine (64 mcg/mL) in 5% dextrose 250 mL
Indication and Important Risk Information
Indication
• Norepinephrine Bitartrate in Dextrose Injection is indicated to raise blood pressure in adult patients with severe, acute hypotension.
Important Risk Information
• Contraindications: None.• Tissue Ischemia: Administration of Norepinephrine Bitartrate in Dextrose Injection to patients who are hypotensive from hypovolemia can result in severe peripheral and visceral vasoconstriction, decreased renal perfusion and reduced urine output, tissue hypoxia, lactic acidosis, and reduced systemic blood flow despite “normal” blood pressure. Address hypovolemia prior to initiating Norepinephrine Bitartrate in Dextrose Injection. Avoid use in patients with mesenteric or peripheral vascular thrombosis, as this may increase ischemia and extend the area of infarction.
Gangrene of the extremities has occurred in patients with occlusive or thrombotic vascular disease or who received prolonged or high dose infusions. Monitor for changes to the skin of the extremities in susceptible patients.
Extravasation of Norepinephrine Bitartrate in Dextrose Injection may cause necrosis and sloughing of surrounding tissue. To reduce the risk of extravasation, infuse into a large vein, check the infusion site frequently for free flow, and monitor for signs of extravasation.
Avoid administration into the veins in the leg in elderly patients.
Emergency Treatment of Extravasation: Infiltrate the ischemic area as soon as possible, using a syringe with a fine hypodermic needle with 5 to 10 mg of phentolamine mesylate in 10 to 15 mL of 0.9% Sodium Chloride Injection in adults.
• Hypotension after Abrupt Discontinuation: Sudden cessation of the infusion rate may result in marked hypotension. When discontinuing the infusion, gradually reduce the infusion rate while expanding blood volume with intravenous fluids.
• Cardiac Arrhythmias: Norepinephrine Bitartrate in Dextrose Injection elevates intracellular calcium concentrations and may cause arrhythmias, particularly in the setting of hypoxia or hypercarbia. Perform continuous cardiac monitoring of patients with arrhythmias.
• Elderly Patients: May be at a greater risk of developing adverse reactions.
• Adverse Reactions: Most common adverse reactions are hypertension and bradycardia.
• Drug Interactions:
- Co-administration of Norepinephrine Bitartrate in Dextrose Injection with monoamine oxidase (MAO) inhibitors or other drugs with MAO-inhibiting properties (e.g., linezolid) or with tricyclic antidepressants can cause severe, prolonged hypertension.
- Anti-diabetics: Norepinephrine Bitartrate in Dextrose Injection can decrease insulin sensitivity and raise blood glucose.
- Concomitant use of Norepinephrine Bitartrate in Dextrose Injection with halogenated anesthetics may lead to ventricular tachycardia or ventricular fibrillation. Monitor cardiac rhythm in patients receiving concomitant halogenated anesthetics.
Please see accompanying full Prescribing Information for Norepinephrine Bitartrate in 5% Dextrose Injection.
