A Homecoming — ZOSYN (piperacillin and tazobactam) Injection
Indications and Select Important Risk Information:
Zosyn is a combination of piperacillin, a penicillin-class antibacterial and tazobactam, a beta-lactamase inhibitor, indicated for the treatment of:
Intra-abdominal infections in adult and pediatric patients 2 months of age and older, Nosocomial pneumonia in adult and pediatric patients 2 months of age and older, Skin and skin structure infections in adults, Female pelvic infections in adults, and Community-acquired pneumonia in adults.
Contraindications
Zosyn is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or beta-lactamase inhibitors.
Warnings and Precautions
Hypersensitivity Adverse Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with Zosyn. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. If an allergic reaction occurs, Zosyn should be discontinued and appropriate therapy instituted.
Please read the accompanying full Indications and Important Risk Information and full Prescribing Information
One source. One contact.
The first and only premix piperacillin and tazobactam injection manufactured by and available from Baxter with one point of contact.
Baxter is the leader in frozen premix IV anti-infectives
Baxter offers practical and innovative products in premix ready-to-use IV formulations, including frozen, which
may increase hospital efficiency while helping to support patient care.
How might your institution benefit from adding Baxter premixes? Choose Zosyn premix for patient safety and
operational efficiency.
Safety
• Standardized concentration may help reduce medication errors associated with compounding preparations1,2
• Barcoded for bedside scanning to help ensure the right patient gets the right medication1
Efficiency
• No admixing or batching required—which may help streamline deployment and save pharmacy time and resources
• Premix medications like Zosyn premix support inventory management and help reduce waste3
Consistent concentration
• A manufacturer-prepared piperacillin and tazobactam injection helps ensure that patients receive a consistent concentration of medication
Value
• Manufacturer-prepared premix medications have a shelf life of 9 to 24 months
Patient care
• Potentially shortening the time between ordering and administration may allow you to spend more time with your patients
Standardized and Consistent
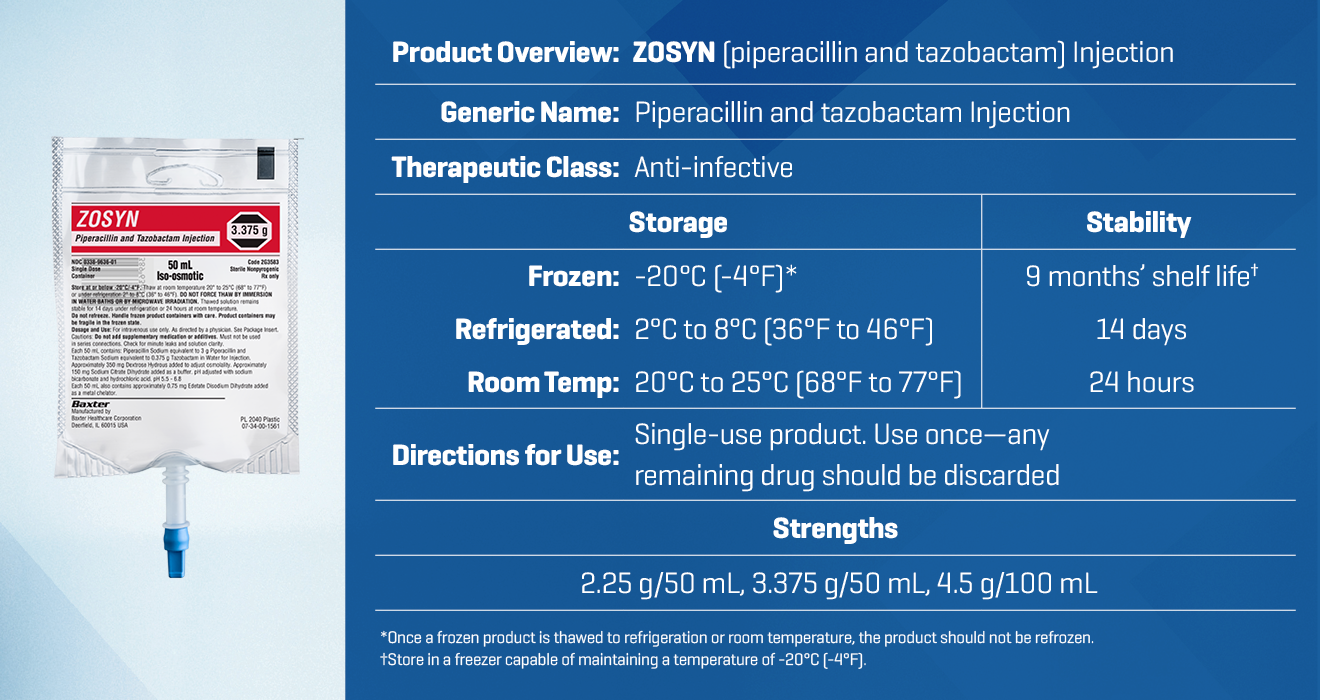
Zosyn premix is available in 3 strengths to support your institution’s needs.
Other ways to order ZOSYN premix:
Visit: Product eCatalog
Phone: 1-888-229-0001
Government Customer Service Number: 1-800-777-2298
Add ZOSYN premix or other frozen premix medications for your facility
Please ask your Baxter sales representative how Zosyn premix can help your healthcare operations.
Don’t have one? Call: 1-888-229-0001
Or visit: Product eCatalog
ZOSYN (piperacillin and tazobactam) Injection
Indications and Important Risk Information
Indications
Zosyn is a combination of piperacillin, a penicillin-class antibacterial and tazobactam, a beta-lactamase inhibitor, indicated for the treatment of:
• Intra-abdominal infections in adult and pediatric patients 2 months of age and older
• Nosocomial pneumonia in adult and pediatric patients 2 months of age and older
• Skin and skin structure infections in adults
• Female pelvic infections in adults
• Community-acquired pneumonia in adults
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Zosyn and other antibacterial drugs, Zosyn should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
Contraindications
Zosyn is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or beta-lactamase inhibitors.
Warnings and Precautions
• Hypersensitivity Adverse Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with Zosyn. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. If an allergic reaction occurs, Zosyn should be discontinued and appropriate therapy instituted.
• Severe Cutaneous Adverse Reactions: Zosyn may cause severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis. If patients develop a skin rash they should be monitored closely and Zosyn discontinued if lesions progress.
• Hemophagocytic Lymphohistiocytosis (HLH): Cases of HLH have been reported in pediatric and adult patients treated with Zosyn. Signs and symptoms of HLH may include fever, rash, lymphadenopathy, hepatosplenomegaly and cytopenia. If HLH is suspected, discontinue Zosyn immediately and institute appropriate management.
• Hematologic Adverse Reactions: Bleeding manifestations have occurred in some patients receiving beta-lactam drugs, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, Zosyn should be discontinued and appropriate therapy instituted. The leukopenia/neutropenia associated with Zosyn administration appears to be reversible and most frequently associated with prolonged administration.
Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy, i.e., ≥ 21 days.
• Central Nervous System Adverse Reactions: As with other penicillins, Zosyn may cause neuromuscular excitability or seizures. Patients receiving higher doses, especially patients with renal impairment may be at greater risk for central nervous system adverse reactions. Closely monitor patients with renal impairment or seizure disorders for signs and symptoms of neuromuscular excitability or seizures.
• Nephrotoxicity in Critically Ill Patients: The use of Zosyn was found to be an independent risk factor for renal failure and was associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs in critically ill patients. Alternative treatment options should be considered in the critically ill population. If alternative treatment options are inadequate or unavailable, monitor renal function during treatment with Zosyn.
• Clostridioides difficile-associated diarrhea (CDAD): CDAD has been reported with use of nearly all antibacterial agents, including Zosyn, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
• Adverse Reactions: The most common adverse reactions (incidence >5%) are diarrhea, constipation, nausea, headache, and insomnia.
• Renal Impairment: In patients with creatinine clearance ≤ 40 mL/min and dialysis patients (hemodialysis and CAPD), the intravenous dose of Zosyn should be reduced to the degree of renal function impairment.
• Drug Interactions:
- Zosyn administration can significantly reduce tobramycin concentrations in hemodialysis patients. Monitor tobramycin concentrations in these patients.
- Probenecid prolongs the half-lives of piperacillin and tazobactam and should not be co-administered with Zosyn unless the benefit outweighs the risk.
- Co-administration of Zosyn with vancomycin may increase the incidence of acute kidney injury. Monitor kidney function in patients receiving Zosyn and vancomycin.
- Monitor coagulation parameters in patients receiving Zosyn and heparin or oral anticoagulants.
- Zosyn may prolong the neuromuscular blockade of vecuronium and other non-depolarizing neuromuscular blockers. Monitor for adverse reactions related to neuromuscular blockade.
Please see accompanying full Prescribing Information for Zosyn.