When the Pressure to Treat Hypotension Rises
Indications and Select Important Risk Information
- Vasopressin in Sodium Chloride Injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
- Contraindications: Vasopressin in Sodium Chloride Injection is contraindicated in patients with a known allergy or hypersensitivity to 8-L-arginine vasopressin.
Please read the accompanying full Indications and Important Risk Information and full Prescribing Information

Product Overview
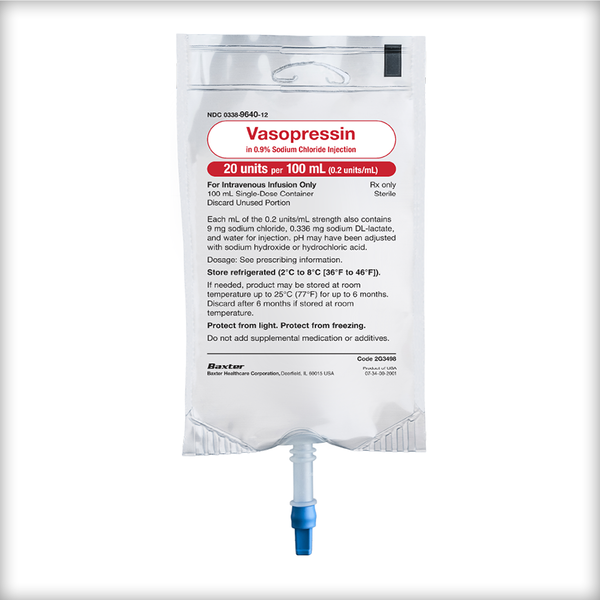
Vasopressin premix from Baxter is a ready-to-use product that delivers the quality of a manufacturer-prepared formulation. Supplied in a closed system GALAXY flexible container that does not require a vented IV set,1 Vasopressin premix is another addition to the Baxter portfolio of high-alert medications that’s ready when you need it.
Vasopressin Premix
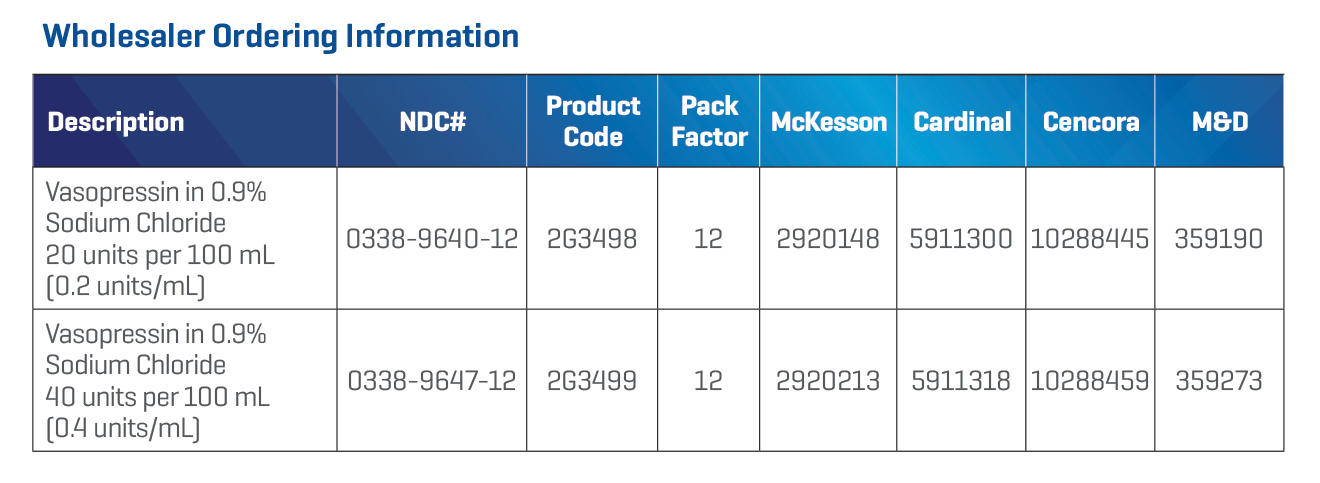
Storage Options for Your Healthcare Facility Needs*
Delivered in a flexible GALAXY IV container
24 months shelf-life when refrigerated
6-month stability at room temperature†
*Product must be stored in a light-protective carton during storage.
†You may receive product labeled with 72-hour room-temperature stability. Vasopressin has since been FDA approved for 6-month room-temperature stability. Please refer to our customer letter for additional information on the 6-month room-temperature stability. Once stored at room temperature, do not place back in the refrigerator.

-
Flexibility—The lightweight, flexible GALAXY container is shatterproof and can be transported from the pharmacy to the point of care without the risk of broken glass
-
Sterility—The sterile, closed-system GALAXY container is collapsible, delivering the full labeled volume of drug solution and does not require a vented IV set.1 Premixed medications in GALAXY container prevent the need for manual admixtures and offer a closed system, reducing the possibility of contamination2
-
Efficiency—Vasopressin is ready for IV administration directly from the GALAXY container. It requires no compounding or transfer to a different container
The GALAXY container is free of latex, PVC, and DEHP1
High-alert medications require special safeguards to reduce the risk of errors.
ISMP and ASHP guidelines recommend using commercially prepared, premixed IV products as a risk-reduction strategy for IV medications3,4
Safety
• Utilizing premix bags with standardized concentration may decrease the likelihood of compounding errors that may be heightened in urgent situations3,4
• Barcoded for bedside scanning to help ensure the right patient gets the right medication4
• Distinctly labeled bag differentiates product strength
Efficiency
• No admixing or batching required—which may help streamline deployment and save pharmacy time and resources
• Premix medications like Vasopressin support inventory management and help reduce waste5
Consistent concentration
• A manufacturer-prepared Vasopressin premix helps ensure that patients receive a consistent concentration of medication4
Vasopressin in 0.9% Sodium Chloride Injection
Indications and Important Risk Information
Indications
- Vasopressin in Sodium Chloride Injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
Important Risk Information
- Contraindications: Vasopressin in Sodium Chloride Injection is contraindicated in patients with a known allergy or hypersensitivity to 8-L-arginine vasopressin.
- Worsening Cardiac Function: A decrease in cardiac index may be observed with the use of vasopressin.
- Reversible Diabetes Insipidus: Patients may experience reversible diabetes insipidus, manifested by the development of polyuria, a dilute urine, and hypernatremia, after cessation of treatment with vasopressin. Monitor serum electrolytes, fluid status, and urine output after vasopressin discontinuation. Some patients may require readministration of vasopressin or administration of desmopressin to correct fluid and electrolyte shifts.
- Adverse Reactions:
– The most common adverse reactions include decreased cardiac output, bradycardia, tachyarrhythmias, hyponatremia, and ischemia (coronary, mesenteric, skin, digital). - Drug Interactions:
– Pressor effects of catecholamines and Vasopressin in Sodium Chloride Injection are expected to be additive.
– Indomethacin may prolong effects of Vasopressin in Sodium Chloride Injection.
– Co-administration of ganglionic blockers or drugs causing SIADH (syndrome of inappropriate antidiuretic hormone secretion) may increase the pressor response.
– Co-administration of drugs causing diabetes insipidus may decrease the pressor response. - Pregnancy: May induce tonic uterine contractions that could threaten the continuation of pregnancy.
Please see accompanying full Prescribing Information for Vasopressin in 0.9% Sodium Chloride Injection.