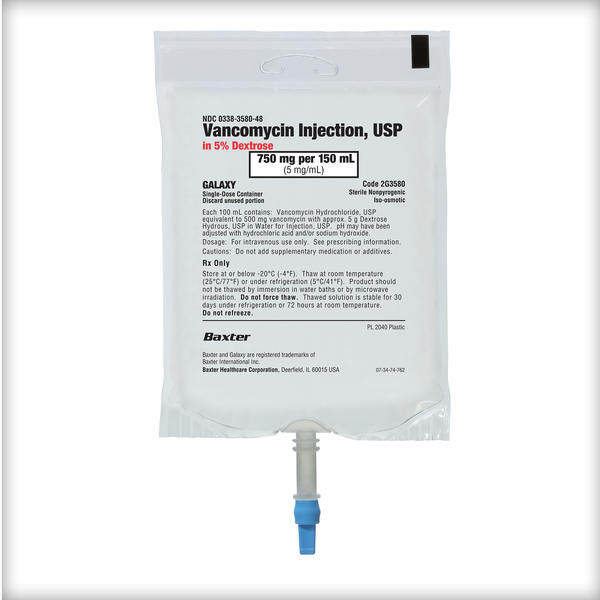
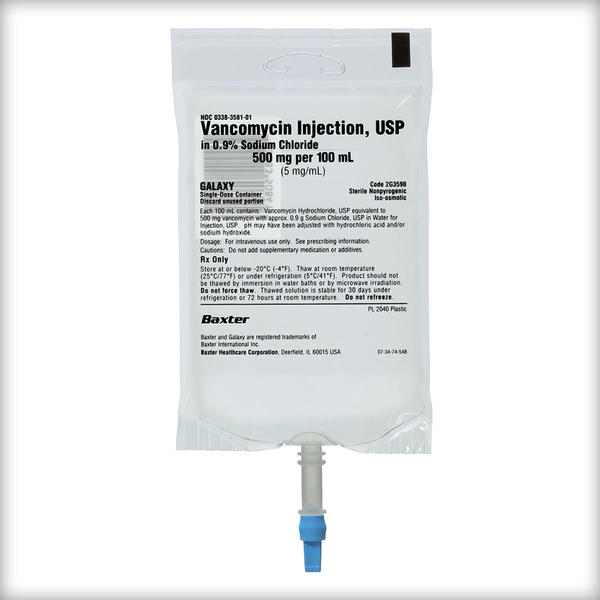
Frozen Premix Vancomycin Injection, USP
Select Indications and Important Risk Information
Vancomycin Injection, USP
Vancomycin is indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant (beta-lactam-resistant) staphylococci. It is indicated for penicillin-allergic patients, for patients who cannot receive or who have failed to respond to other drugs, and for infections caused by vancomycin-susceptible organisms that are resistant to other antimicrobial drugs. Vancomycin is effective in the treatment of Infective Endocarditis, Septicemia, Skin and Skin Structure Infections, Bone Infections, and Lower Respiratory Tract Infections.
Contraindications: Vancomycin is contraindicated in patients with known hypersensitivity to this antibiotic. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
Please read the accompanying full Indications and Important Risk Information and full Prescribing Information

Vancomycin Injection, USP
When it comes to high-volume medications like IV vancomycin, we know that more options in a premix formulation mean you can treat more patients with the safety of a premix, with more efficiency and less waste.1-3
That’s why Baxter has added 2 strengths to expand its offering of Vancomycin Injection in Dextrose to a total of 5 doses, to help you support patient care.
Vancomycin Injection, USP in 0.9% Sodium Chloride
Contact your representative directly
By Phone
Government Customer Service
Baxter is the leader in frozen premix IV anti-infectives. Premix Vancomycin for patient safety considerations and operational efficiency.
Safety
- Standardized concentration may help reduce medication errors associated with compounding
preparation1,2
- Barcoded for bedside scanning to help ensure the right patient gets the right medication2
Efficiency
- No admixing or batching required—which may help streamline deployment and save pharmacy time and resources
- Premix medications like Vancomycin support inventory management and help reduce waste3
Consistent concentration
A manufacturer-prepared vancomycin injection helps ensure that patients receive a consistent concentration of medication2
Add Vancomycin to your Premix portfolio
Reach out to your Baxter sales representative. Don’t have one? Call: 1.888.229.0001
Or visit: Product eCatalog
Baxter offers practical and innovative products in premix ready-to-use IV formulations, including frozen, which may increase hospital efficiency while helping to support patient care.
How might your institution benefit from adding additional Baxter premixes?
- SAFETY: Reducing the potential for medication errors due to compounding1,2
- EFFICIENCY: Improved workflow by allowing on-demand availability of medications
- VALUE: Manufacturer-prepared premix medications have a shelf life of 9 to 24 months
- PATIENT CARE: Potentially shortening the time between ordering and administration may allow you to spend more time with your patients
Vancomycin Injection, USP
Indications and Important Risk Information
Indications
Vancomycin is indicated for the treatment of serious or severe infections caused by susceptible strains of methicillin-resistant (beta-lactam-resistant) staphylococci. It is indicated for penicillin-allergic patients, for patients who cannot receive or who have failed to respond to other drugs, and for infections caused by vancomycin-susceptible organisms that are resistant to other antimicrobial drugs.
Vancomycin is effective in the treatment of:
- Infective Endocarditis
- staphylococcal endocarditis
- endocarditis caused by Streptococcus viridans or S. bovis, alone or in combination with an aminoglycoside
- endocarditis caused by enterococci (e.g., E. faecalis), only in combination with an aminoglycoside
- diphtheroid endocarditis
- early-onset prosthetic valve endocarditis caused by S. epidermidis or diphtheroids in combination with either rifampin, an aminoglycoside, or both - Septicemia
- Skin and Skin Structure Infection
- Bone Infections
- Lower Respiratory Tract Infections
To reduce the development of drug-resistant bacteria and maintain the effectiveness of vancomycin and other antibacterial drugs, vancomycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
- Contraindications: Vancomycin is contraindicated in patients with known hypersensitivity to this antibiotic. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
- Infusion Reactions: Rapid bolus administration (e.g., over several minutes) may be associated with exaggerated hypotension, including shock, and, rarely, cardiac arrest.
During or soon after rapid infusion of vancomycin, patients may develop anaphylactoid reactions, including hypotension, wheezing, dyspnea, urticaria, or pruritus. Rapid infusion may also cause flushing of the upper body (“vancomycin infusion reaction”) or pain and muscle spasm of the chest and back.
Vancomycin should be administered over a period of not less than 60 minutes. Stopping the infusion usually results in prompt cessation of these reactions. - Nephrotoxicity: Systemic vancomycin exposure may result in acute kidney injury (AKI). The risk of AKI increases as systemic exposure/serum levels increase. Monitor renal function in all patients; especially with underlying renal impairment, with co-morbidities, and receiving concomitant therapy with a known nephrotoxic drug.
- Ototoxicity: It may be transient or permanent. It has been reported mostly in patients who have been given excessive doses, who have an underlying hearing loss, or who are receiving concomitant therapy with another ototoxic agent, such as an aminoglycoside. Vancomycin should be used with caution in patients with renal insufficiency.
- Severe Dermatologic Reactions: Toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) have been reported. Cutaneous signs or symptoms reported include skin rashes, mucosal lesions, and blisters. Discontinue Vancomycin Injection at the first appearance of signs and symptoms of TEN, SJS, DRESS, AGEP, or LABD. Dosage of vancomycin must be adjusted for patients with renal dysfunction.
- Clostridioides difficile associated diarrhea (CDAD): May range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
- Hemorrhagic Occlusive Retinal Vasculitis: Including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or the intravitreal route have not been established.
- Adverse Reactions: Not already mentioned above, patients have been reported to have neutropenia, phlebitis, drug fever, nausea, chills, and vasculitis in association with administration of vancomycin.
- Drug Interactions:
- Anesthetic Agents: Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing and anaphylactoid reactions.
- Monitor renal function in patients receiving vancomycin and concurrent and/or sequential systemic or topical use of other potentially neurotoxic and/or nephrotoxic drugs, such as amphotericin B, aminoglycosides, bacitracin, polymyxin B, colistin, viomycin, or cisplatin.
Please see accompanying full Prescribing Information for Vancomycin Injection, USP.