Amino Acid Injections
A broad range of sulfite-free amino acid injections. Concentrations up to 20% in a wide array of bag sizes to meet critical needs.

Amino acid options to support protein needs with less fluid.
Baxter offers a broad array of amino acid options to nourish your pediatric and adult patients.
Indications
Premasol 10% sulfite-free (amino acid) injection is indicated for the nutrition support of infants (including those of very low birth weight) and young children requiring TPN via central or peripheral infusion routes. Click here for full Indication(s) and Important Risk Information and full Prescribing Information for Premasol.
15% CLINISOL sulfite-free (Amino Acid) Injection is indicated as an adjunct in the offsetting of nitrogen loss or in the treatment of negative nitrogen balance in patients where: (1) the alimentary tract cannot or should not be used, (2) gastrointestinal absorption of protein is impaired, or (3) metabolic requirements for protein are substantially increased, as with extensive burns. Please read the accompanying full Indication(s) and Important Risk Information and full Prescribing Information for Clinisol.
TRAVASOL (amino acid) Injection is indicated as a source of amino acids for patients requiring parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated. TRAVASOL may be used to treat negative nitrogen balance in patients. Please read the accompanying full Indication(s) and Important Risk Information and full Prescribing Information for Travasol.
20% PROSOL (amino acids) Injection is indicated as a source of amino acids for patients requiring parent eral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated. PROSOL may be used to treat negative nitrogen balance in patients. Please read the accompanying full Indication(s) and Important Risk Information and full Prescribing Information for Prosol.
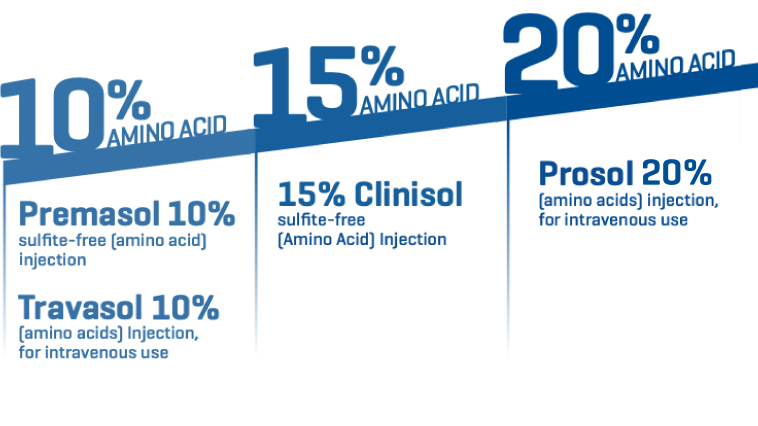
Amino acid options to support protein needs with less fluid.
Baxter offers a broad array of amino acid options to nourish your pediatric and adult patients, including:
• Premasol 10% sulfite-free (amino acid) injection, indicated for pediatric patients
• Travasol 10% (amino acids) Injection, for intravenous use
• 15% Clinisol sulfite-free (Amino Acid) Injection
• Prosol 20% (amino acids) injection, for intravenous use
Premasol 10% Solubility Data
We have established solubility data for PN solutions containing Premasol 10% sulfite-free (amino acid) injection.
Request More Information About Amino Acid Injections
Explore Products for Individualized Clinical Nutrition
Indications and Important Safety Information
PREMASOL 10% sulfite-free (amino acid) injection Indications and Important Risk Information
Indications
PREMASOL 10% sulfite-free (amino acid) injection is indicated for the nutrition support of Infants (including those of very low birth weight) and young children requiring TPN via central or peripheral infusion routes. Parenteral nutrition with PREMASOL injection is indicated to prevent nitrogen and weight loss or treat negative nitrogen balance in infants and young children where: (1) the alimentary tract, by the oral, gastrostomy, or jejunostomy route, cannot or should not be used or adequate protein intake is not feasible by these routes, (2) gastrointestinal absorption of protein is impaired, or (3) protein requirements are substantially increased, as with extensive burns. Dosage, route of administration, and concomitant infusion of non-protein calories are dependent on various factors, such as nutritional and metabolic status of the patient, anticipated duration of parenteral nutrition support, and vein tolerance.
Important Risk Information
- PREMASOL 10% injection is contraindicated in patients with untreated anuria, hepatic coma, inborn errors of amino acid metabolism, including those involving branched chain amino acid metabolism such as maple syrup urine disease and isovaleric acidemia, or hypersensitivity to one or more amino acids in the solution.
- This injection is for compounding only, not for direct infusion
- Safe, effective use of parenteral nutrition requires know ledge of nutrition as well as clinical expertise in recognition and treatment of the complications which can occur.
- Frequent evaluation and laboratory determinations are necessary for proper monitoring of parenteral nutrition. Depending on the patient’s conditions or disease state, additional electrolyte supplementation may be required.
- Fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states, or pulmonary edema can occur with IV administration.
- Administration of amino acids in patients with impaired renal function or gastrointestinal bleeding may augment an already elevated blood urea nitrogen. Do not infuse amino acids in patients with azotemia.
- Administration of amino acid solutions to a patient with hepatic insufficiency may result in plasma amino acid imbalances, hyperammonemia, prerenal azotemia, stupor and coma.
- It is essential that blood ammonia be measured frequently in infants. Hyperammonemia in the syndrome is caused by genetic metabolic defects and is sometimes associated, although not necessarily in a causal relationship, with mental retardation. This reaction appears to be dose related and is more likely to develop during prolonged therapy. Should symptoms of hyperammonemia develop, amino acid administration should be discontinued and patient’s clinical status reevaluated.
- This product contains aluminum that may be toxic with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
- Strongly hypertonic nutrient solutions should be administered via an intravenous catheter placed in a central vein, preferably the superior vena cava.
- Special care must be taken when giving hypertonic dextrose to patients with diabetes or impaired glucose tolerance. To prevent hyperglycemia in such patients, insulin may be required.
- The final infusate should be inspected for cloudiness or precipitation immediately after mixing, prior to administration, and periodically during administration.
- Adverse reactions reported in clinical studies were: water weight gain, edema, increase in BUN, and mild acidosis. Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypovolemia. Phosphorus deficiency may lead to impaired tissue oxygenation and acute hemolytic anemia. Relative to calcium, excessive phosphorous intake can precipitate hypocalcemia with cramps, tetany, and muscular hyperexcitability. If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic counter measures and save the remainder of the fluid for examination, if deemed necessary.
- Protect from light until immediately prior to use.
Please click here to see accompanying full Prescribing Information for PREMASOL 10%
TRAVASOL (amino acid) Injection Indications and Important Risk Information
Indications
TRAVASOL (amino acid) Injection is indicated as a source of amino acids for patients requiring parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated. TRAVASOL may be used to treat negative nitrogen balance in patients.
Important Risk Information
- Contraindicated in patients with known hypersensitivity to one or more amino acids, inborn errors of amino acid metabolism, and pulmonary edema or acidosis due to low cardiac output.
- Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving PN. In some cases, fatal outcomes due to pulmonary embolism have occurred.
- Hypersensitivity reactions including anaphylaxis have been reported with parenteral nutrition solutions containing TRAVASOL. Stop the infusion immediately and treat patient accordingly if any signs or symptoms of a hypersensitivity reaction develop.
- Patients who require PN are at high risk of infections because the nutritional components of these solutions can support microbial growth. Infection and sepsis may also occur as a result of the use of intravenous catheters to administer PN.
- Refeeding severely undernourished patients may result in refeeding syndrome, characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic.
- Administration of PN solutions containing dextrose in patients with diabetes mellitus, impaired glucose tolerance may worsen hyperglycemia. Neonates, especially premature infants with low birth weight, are at increased risk of developing hypo- or hyperglycemia and therefore need close monitoring during treatment with intravenous glucose solutions to ensure adequate glycemic control in order to avoid potential long term adverse effects.
- TRAVASOL must be diluted and used as an admixture with or without dextrose, electrolytes and/or lipid emulsion. It is not for direct intravenous infusion. Solutions containing more than 5% dextrose or with an osmolarity of 900 mOsm/L or greater must be infused through a central catheter. The infusion of hypertonic nutrient injections into a peripheral vein may result in vein irritation, vein damage, and/or thrombosis.
- Hepatobiliary disorders are known to develop in some patients without preexisting liver disease who receive parenteral nutrition, including cholecystitis, cholelithiasis, cholestasis, hepatic steatosis, fibrosis and cirrhosis, possibly leading to hepatic failure. Increase in blood ammonia levels and hyperammonemia may occur in patients receiving amino acid solutions, including TRAVASOL.
- TRAVASOL contains no more than 25 mcg/L of aluminum. However, with prolonged parenteral administration in patients with renal impairment, the aluminum contained in TRAVASOL may reach toxic levels. Preterm infants are at a greater risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Patients with renal impairment, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day, accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- Parenteral Nutrition Associated Liver Disease (PNALD) ; increased risk in patients who receive parenteral nutrition for extended periods of time, especially preterm infants; monitor liver function tests, if patients abnormalities occur consider discontinuation or dosage reduction.
- Electrolyte Imbalance and Fluid Overload: patients with cardiac insufficiency or renal impairment may require adjustment of fluid, protein, and electrolyte content.
- Monitor fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count and coagulation parameters throughout treatment. If electrolyte levels are severely elevated, stop PN containing TRAVASOL until levels have been corrected.
- Prior to infusion, visually inspect the diluted PN solution containing TRAVASOL for particulate matter. The solution should be clear and there should be no precipitates. A slight yellow color of the amino acid does not alter the quality and efficacy of this product. If lipid has been added, ensure the emulsion has not separated. Separation of the emulsion can be visibly identified. Discard the admixture if precipitates or separation are observed.
- Protect the admixed parenteral nutrition solutions from light.
Please click here to see accompanying full Prescribing Information for TRAVASOL.
15% CLINISOL sulfite-free (Amino Acid) Injection Indications and Important Risk Information
Indications
15% CLINISOL sulfite-free (Amino Acid) Injection is indicated as an adjunct in the offsetting of nitrogen loss or in the treatment of negative nitrogen balance in patients where: (1) the alimentary tract cannot or should not be used, (2) gastrointestinal absorption of protein is impaired, or (3) metabolic requirements for protein are substantially increased, as with extensive burns.
Important Risk Information
- Contraindicated in patients with hypersensitivity to one or more amino acids, severe liver disease or hepatic coma, Anuria and Metabolic disorders involving impaired nitrogen utilization.
- Because of the potential for life threatening events, caution should be taken to ensure that precipitates have not formed in any parenteral nutrition admixture.
- This injection is for compounding only, not for direct infusion.
- Administration of amino acid solutions at excessive rates or to patients with hepatic insufficiency may result in plasma amino acid imbalances, hyperammonemia, prerenal azotemia, stupor and coma. If hyperammonemia develops, discontinue the amino acid administration and reevaluate the patient’s clinical status.
- This product contains aluminum that may be toxic with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amount of calcium and phosphate solutions, which contain aluminum.
- The administration of 15% Clinisol Injection as part of the total parenteral nutrition with large volumes of hyperosmotic fluids requires periodic monitoring for signs of hyperosmolarity, hyperglycemia, glycosuria, hypertriglyceridemia and volume overload. Initiation and termination of TPN infusion must be gradual to permit adjustment of endogenous insulin release.
- During parenteral nutrition with concentrated dextrose and amino acid solutions, essential fatty acid deficiency syndrome may develop and therefore plasma lipids should be monitored. This syndrome may be prevented or corrected by treatment with intravenous fat emulsions.
- Frequent clinical evaluations and laboratory determinations are necessary for proper monitoring during administration.
- Total parenteral nutrition therapy may include multiple vitamins, trace elements and additional electrolytes. Potentially incompatible ions such as calcium and phosphate may be added to alternate infusate containers to avoid precipitation.
- Local adverse reactions consisting of a warm sensation, erythema, phlebitis and thrombosis at the infusion site have occurred with peripheral intravenous infusion of amino acids. Generalized flushing, fever and nausea have been reported during peripheral infusions of amino acid solutions.
- The following metabolic complications have been reported with administration of TPN: metabolic acidosis and alkalosis, hypophosphatemia, hypocalcemia, osteoporosis, glycosuria, hyperglycemia, hyperosmolar nonketotic states and dehydration, rebound hypoglycemia, osmotic diuresis and dehydration, elevated liver enzymes, hypo- and hypervitaminosis, electrolyte imbalances, hyperammonemia, coma and death.
Please click here to see accompanying full Prescribing Information for CLINISOL.
PROSOL (amino acids) injection, for intravenous use Indications and Important Risk Information
Indications
PROSOL is indicated as a source of amino acids for patients requiring parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated. PROSOL may be used to treat negative nitrogen balance in patients.
Important Risk Information
- Contraindicated in patients with known hypersensitivity to one or more amino acids, inborn errors of amino acid metabolism and patients with pulmonary edema or acidosis due to low cardiac output.
- Pulmonary vascular precipitates causing pulmonary vascular emboli and pulmonary distress have been reported in patients receiving PN. In some cases, fatal outcomes due to pulmonary embolism have occurred.
- Hypersensitivity reactions including anaphylaxis have been reported with PN solutions containing PROSOL. Stop the infusion immediately and treat patient accordingly if any signs or symptoms of a hypersensitivity reaction develop.
- Patients who require PN are at high risk of infections because the nutritional components of these solutions can support microbial growth. Infection and sepsis may also occur as a result of the use of intravenous catheters to administer PN.
- Refeeding severely undernourished patients may result in refeeding syndrome, characterized by the intracellular shift of potassium, phosphorus, and magnesium as the patient becomes anabolic.
- Administration of PN solutions containing dextrose in patients with diabetes mellitus, impaired glucose tolerance may worsen hyperglycemia.
- PROSOL must be diluted and used as an admixture with or without dextrose, electrolytes and/or lipid emulsion. It is not for direct intravenous infusion. Solutions containing more than 5% dextrose or with an osmolarity of 900 mOsm/L or greater must be infused through a central catheter.
- Hepatobiliary disorders are known to develop in some patients without preexisting liver disease who receive PN, including cholecystitis, cholelithiasis, cholestasis, hepatic steatosis, fibrosis and cirrhosis, possibly leading to hepatic failure. Increase in blood ammonia levels and hyperammonemia may occur in patients receiving amino acid solutions, including PROSOL.
- PROSOL contains no more than 25 mcg/L of aluminum. However, with prolonged parenteral administration in patients with renal impairment, the aluminum contained in PROSOL may reach toxic levels. Preterm infants are at a greater risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum. Patients with renal impairment, including preterm infants, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day, accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
- Parenteral Nutrition Associated Liver Disease: increased risk in patients who receive parenteral nutrition for extended periods of time, especially preterm infants; monitor liver function tests, if abnormalities occur consider discontinuation or dosage reduction
- Electrolyte Imbalance and Fluid Overload: patients with cardiac insufficiency or renal impairment may require adjustment of fluid, protein and electrolyte content.
- Adverse reactions include pulmonary vascular emboli, hypersensitivity reactions infection, refeeding syndrome, hyperglycemia and hyperosmolar hyperglycemic state, vein thrombosis, hepatobiliary disorders, aluminum toxicity, parenteral nutrition associated liver disease, electrolyte imbalance and fluid overload. The following metabolic complications have been reported with parenteral amino acid products: metabolic acidosis, alkalosis, rebound hyperglycemia, osmotic diuresis and dehydration, hypo and hypervitaminosis.
- Monitor fluid and electrolyte status, serum osmolarity, blood glucose, liver and kidney function, blood count and coagulation parameters throughout treatment. If electrolyte levels are severely elevated, stop PN containing PROSOL until levels have been corrected.
- Neonates, especially premature infants with low birth weight, are at increased risk of developing hypo- or hyperglycemia and therefore need close monitoring during treatment with intravenous glucose solutions to ensure adequate glycemic control in order to avoid potential long term adverse effects
- Prior to infusion, visually inspect the diluted PN solution containing PROSOL for particulate matter. The solution should be clear and there should be no precipitates. A slight yellow color does not alter the quality and efficacy of this product. If lipid has been added, ensure the emulsion has not separated. Separation of the emulsion can be visibly identified. Discard the admixture if precipitates or separation are observed.
- Protect the admixed parenteral nutrition solution from light.
Please click here to see accompanying full Prescribing Information for PROSOL.







