Clinolipid (Lipid Injectable Emulsion) for Intravenous Use
Olive oil-based intravenous lipid emulsion (ILE) rich in immune neutral omega-9 fatty acids.1
Biologic studies have demonstrated immune neutral properties of omega-9 fatty acids.2-5 Immune neutral benefits have not been established in clinical studies.

Indication and Select Risk Information
Indication
CLINOLIPID injection is indicated in adults and pediatric patients, including term and preterm neonates as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
Select Important Risk Information
- The use of CLINOLIPID injection is contraindicated in patients with the following:
- Known hypersensitivity to egg, soybean, peanut, or any of the active or inactive ingredients.
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
- Parenteral Nutrition-Associated Liver Disease (PNALD): Increased risk in patients who receive parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests: if abnormalities occur, consider discontinuation or dosage reduction.
Click here for full Indication(s) and Important Risk Information and accompanying full Prescribing Information for CLINOLIPID.
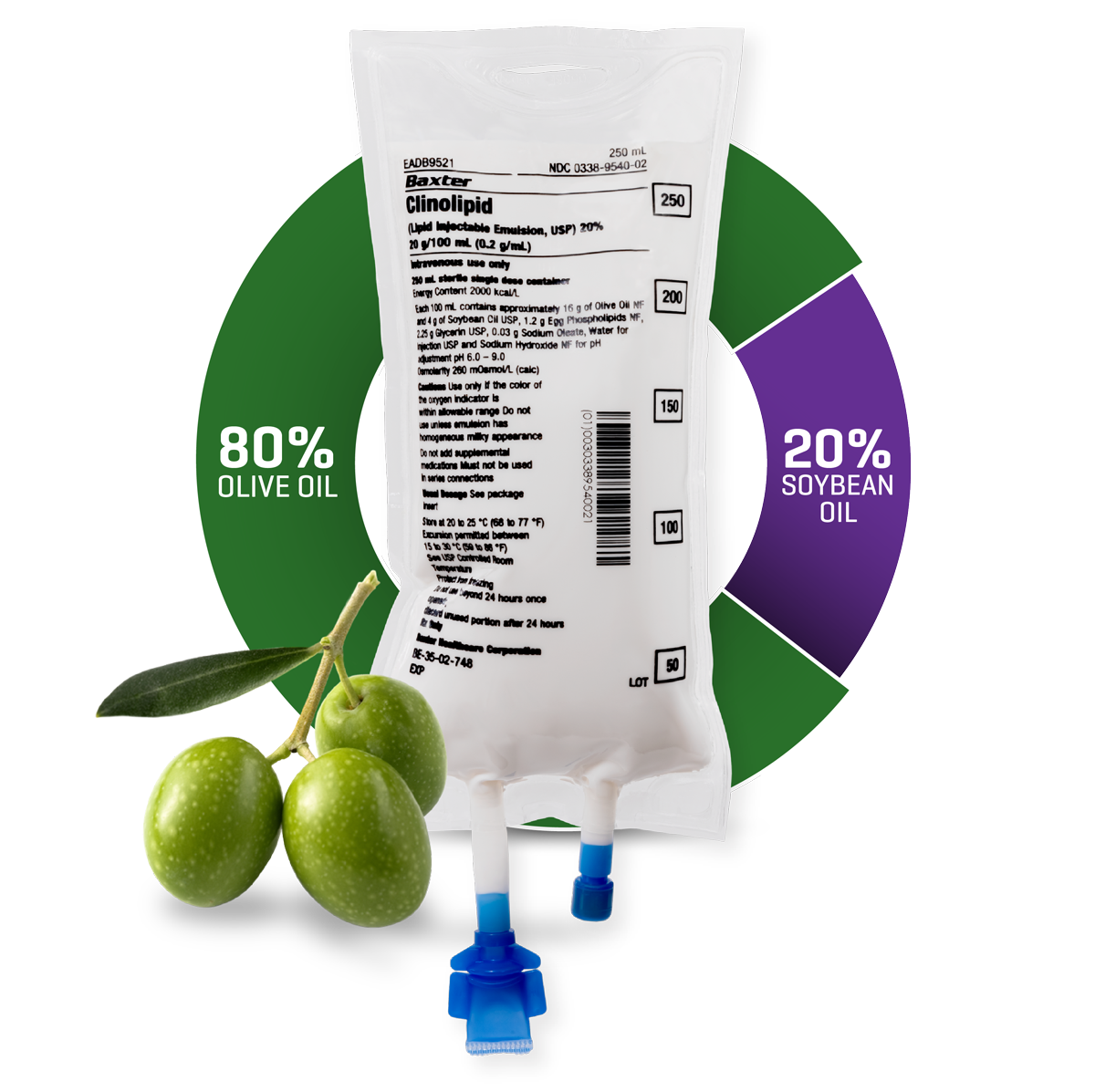
Discover the Versatility of Clinolipid Every Age, Every Day1,6
From neonates to adults, olive oil-based Clinolipid can be your first choice for providing a daily source of essential fatty acids and energy1—with 20% soybean oil, Clinolipid has the lowest amount of any mixed-oil ILE.1,7,8
Help Determine the Clinolipid Dose for Your Patients, from Neonates to Adults1,6
The maximum daily dose of CLINOLIPID should be based on individual total nutritional requirements and patient tolerance, please refer to recommended pediatric and adult dosage. Please refer to the CLINOLIPID Dosing Information regarding initial (first 30 min) and maximum infusion rates.1
Essential Fatty Acid Contribution Calculator
(ALA) a-Linolenic acid
The maximum daily dose of CLINOLIPID should be based on individual total nutritional requirements and patient tolerance, please refer to recommended pediatric and adult dosage. Please refer to the CLINOLIPID Dosing Information regarding initial (first 30 min) and maximum infusion rates.
Clinolipid Dosing Information

The maximum daily dose of CLINOLIPID should be based on individual total nutritional requirements and patient tolerance.
Contained in Clinolipid
| Lipid - 0.2 g/mL | Kcal - 2000 kcal/L | LA - 35.8 mg/mL | ALA - 4.7 mg/mL |
Recommended Pediatric and Adult Dosage and Infusion Rate
| Age | Nutritional Requirements | Direct Infusion Rate* | |
| Recommended Initial Dosage and Maximum Dosage | Initial | Maximum | |
| Birth to 2 years of age (including preterm and term neonates**) [see Warnings & Precautions (5.1) in Prescribing Information] |
Initial 0.5 to 1 g/kg/day not to exceed 3 g/kg/day*** | 0.1 to 0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.75 mL/kg/hour |
| Pediatric patients 2 to less than 12 years of age | Initial 1 to 2 g/kg/day not to exceed 3 g/kg/day*** | 0.2 to 0.4 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.75 mL/kg/hour |
| Pediatric patients 12 to 17 years of age | Initial 1 g/kg/day not to exceed 3 g/kg/day*** | 0.2 to 0.4 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes | 0.75 mL/kg/hour |
| Adults | 1 to 1.5 g/kg/day not to exceed 2.5 g/kg/day*** | 0.2 mL/kg/hour for the first 15 to 30 minutes; if tolerated, gradually increase to the required rate after 30 minutes | 0.5 mL/kg/hour |
*CLINOLIPID Pharmacy Bulk Package is not intended for direct intravenous administration.
**The neonatal period is defined as including term, post-term, and preterm newborn infants. The neonatal period for term and post-term infants is the day of birth plus 27 days. For preterm infants, the neonatal period is defined as the day of birth through the expected age of delivery plus 27 days (i.e., 44 weeks post-menstrual age).
***Daily dosage should also not exceed a maximum of 60% of total energy requirements [See Overdosage (10) in Prescribing Information].
Please see accompanying Prescribing Information for additional Dosage & Administration information.
Supporting Pediatric PN Admixtures
Clinolipid is a well-tolerated, safe and effective source of energy and essential fatty acids that promotes adequate growth and development in neonatal and pediatric patients requiring parenteral nutrition.1

Less Soybean Oil
With 20% soybean oil, Clinolipid has the lowest amount of any mixed-oil ILE.1,7,8

Rich in Omega-9 Monounsaturated Fatty Acid (MUFA)
Clinolipid is rich in omega-9 oleic acid, the most prevalent fatty acid in breast milk.1,9

Extensive PN Admixture Compatibility
From neonates to adults, Clinolipid can be your standard choice ILE supported by extensive PN Admixture compatibility.1,10

Minimizes the decline in postnatal Arachidonic Acid (ARA) levels1,11
ARA is a fatty acid. Fatty acids play an important role in infant growth and development.1,12
Select Important Risk Information
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation.
Click here for full Indication(s) and Important Risk Information and accompanying full Prescribing Information for CLINOLIPID.
Rethink Your Lipid Choice

Fatty Acid Composition
Lipids provide a dense source of energy and essential fatty acids.
Omega-9 (olive oil) is a monounsaturated fatty acid (MUFA).
Omega-6 (soybean oil) is a polyunsaturated fatty acid (PUFA).
The omega-3 and omega-6 fatty acid ratio in Clinolipid injection has not been shown to improve clinical outcomes compared to other intravenous lipid emulsions.

Clinolipid Composition
Clinolipid’s largest component is olive oil, which is rich in omega-9 oleic acid.1,8 Oleic acid is a MUFA that may have immune neutral effects.1-5
Biologic studies have demonstrated immune neutral properties of omega-9 fatty acids. Immune neutral benefits have not been established in clinical studies.
Clinolipid is 64% oleic acid by composition and contains the most MUFA of any mixed-oil ILE available in the United States.1,8
Clinolipid injection contains soybean oil to provide essential fatty acids.1

Access PN Admixture Stability Information
Explore Clinical Nutrition Products
CLINOLIPID (Lipid Injectable Emulsion) for intravenous use Indication and Important Risk Information
Indication
CLINOLIPID injection is indicated in adults and pediatric patients, including term and preterm neonates as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
Important Risk Information
- The use of CLINOLIPID injection is contraindicated in patients with the following:
- Known hypersensitivity to egg, soybean, peanut, or any of the active or inactive ingredients.
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
- Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation.
- Parenteral Nutrition-Associated Liver Disease (PNALD): Increased risk in patients who receive parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests: if abnormalities occur, consider discontinuation or dosage reduction.
- Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
- Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, Hypertriglyceridemia, and Essential Fatty Acid Deficiency (EFAD): Monitor for signs and symptoms; monitor laboratory parameters.
- Ensure aseptic techniques are used for catheter placement, catheter maintenance, and preparation and administration of CLINOLIPID.
- If signs or symptoms of fat overload syndrome occur, stop CLINOLIPID.
- To prevent complications from Refeeding Syndrome, closely monitor severely malnourished patients and slowly increase their nutrient intake.
- Measure serum triglycerides before the start of infusion and regularly throughout treatment. If triglyceride levels are above 400 mg/dL in adults, stop the CLINOLIPID infusion and monitor serum triglyceride levels to avoid clinical consequences of hypertriglyceridemia.
- Laboratory testing using the triene to tetraene ratio may not be adequate to diagnose EFAD, and assessment of individual fatty acid levels may be needed.
- Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates. CLINOLIPID injection contains no more than 25 mcg/L of aluminum.
- Most common (≥5%) adverse drug reactions from clinical trials in adults were nausea and vomiting, hyperlipidemia, hyperglycemia, hypoproteinemia, and abnormal liver function tests.
- Most common (≥5%) adverse reactions from clinical trials in pediatric patients were hyperbilirubinemia, patent ductus arteriosus, anemia, gastroesophageal reflux disease, bradycardia, feeding intolerance, neonatal intraventricular hemorrhage, increased alkaline phosphatase, atrial septal defect, hyponatremia, sepsis, and infantile apnea.
- The anticoagulant activity of coumarin derivatives, including warfarin, may be counteracted.
Click here for accompanying full Prescribing Information for CLINOLIPID.






