Ready to Go the Distance. Ready to Respond.
Built to withstand disruption that no one sees coming.
With one of the most expansive and agile manufacturing networks in the IV solutions industry, Baxter’s supply chain is built to flex, adapt and deliver — supporting consistent care for patients and confidence for our partners no matter what lies ahead.
We have the capabilities to face supply chain challenges and come through stronger and with more insight.
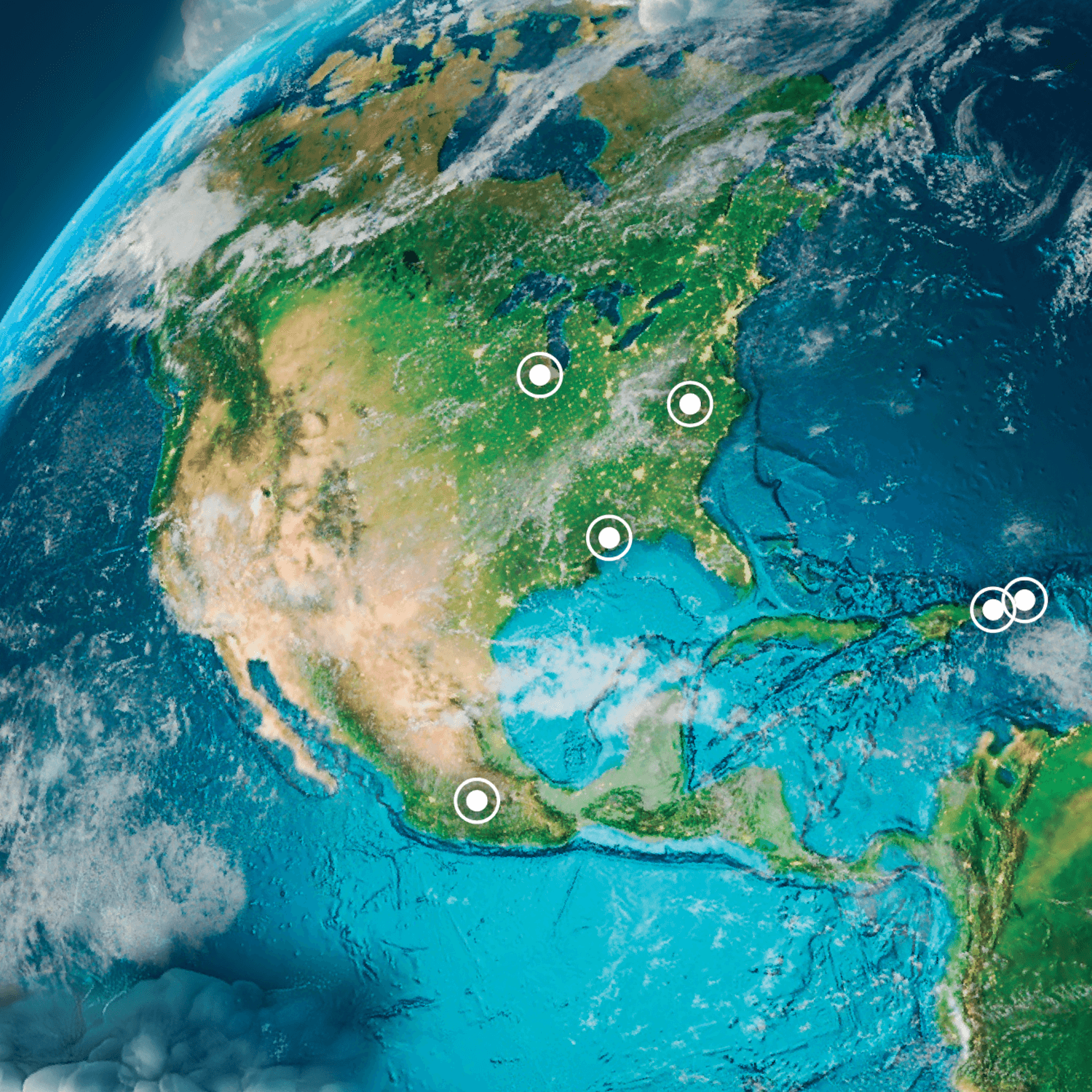
95% of Baxter IV fluids are manufactured for the U.S. in North America across six IV plants,1 building robust resilience and redundancy into the U.S. supply chain.
These six plants are part of the broadest global manufacturing network in the industry.2 All contain satellite phones and generators that can serve as backup in case of power or communications disruption.
Baxter is proud to have received a Resiliency Badge from the Healthcare Industry Resilience Collaborative (HIRC) in both 2023 and 2024.
When forces outside our control put our supply chain to the test, we’re ready.

Activating with purpose
The COVID-19 pandemic and 2024’s Hurricane Helene posed major challenges. We rose to the occasion by initiating a Crisis Control Committee to activate our global network of plants and personnel across the entire organization toward a single goal: recovery.
From disruption to delivery in record time
Within three weeks of the manufacturing disruption caused by Hurricane Helene, hundreds of planes were delivering products from Baxter sites in Asia and Europe, with all imports authorized by the FDA. Within three months, we were able to remove all allocations so our customers could resume standard IV practices for their patients.

Leveraging our experience and learnings
Millions of hours invested in disaster recovery over the last several years have provided us with the operational expertise to overcome challenges. Faced with a spike in demand or a natural disaster, we have the agile system in place to help respond to it.