When the pace of your pharmacy heats up, Frozen Premix Medications help you stay cool
Find your chill with frozen premix
Frozen Premix IV Medications, only from Baxter
Frozen premix IV medications from Baxter ship frozen, but you can put them directly in the fridge so they're ready when you need them. Of course, you also have the option to store them frozen.* It all adds up to the safety and reliability of a premix in a formulation that brings some chill to your workflow.
* Please see respective product prescribing information for full storage and refrigeration/room temperature stability information. Once a frozen product is thawed, it should not be refrozen.
Baxter is the leader in frozen premix IV medications
Order direct from Baxter
Visit: Product eCatalog
Phone: 1-888-229-0001
Government Customer Service Number: 1-800-777-2298
Efficiency
Frozen Premixes help increase operational efficiencies in the pharmacy by requiring fewer preparation steps.
Safety
Frozen premixes deliver consistent drug concentrations, which may help minimize medication errors associated with compounding.1,2
Barcoded labels on Baxter frozen premixes allow for bedside scanning to help ensure the right patient gets the right medication.1
Managing Frozen Premix Medications
Fridge or freezer? You choose. Baxter offers storage and thawing options to fit your needs.
• Temperature control equipment for storage and passive thawing at no up-front cost
• Thawing units for on-demand thawing
• Thawing racks for room temperature thawing
Add Frozen Premix to your healthcare facility
Reach out to your Baxter sales representative. Don't have one? Call: 1.888.229.0001
Order through Baxter eServices
Or visit: eCatalog
Government Customer Service Number: 1-800-777-2298
Featured Frozen Premix Products
Baxter frozen premix IV medications help your healthcare facility meet the needs of your patients.
Please see accompanying Indications, Important Risk Information, and link to the Prescribing Information for each of these products below
Vancomycin Injection, USP
Vancomycin Injection, USP in 5% Dextrose
500 mg/100 mL (5 mg/mL) in single dose GALAXY container
750 mg/150 mL (5 mg/mL) in single dose GALAXY container
1 g/200 mL (5 mg/mL) in single dose GALAXY container
1.25 g/250 mL (5 mg/mL) in single dose GALAXY container
1.5 g/300 mL (5 mg/mL) in single dose GALAXY container
Vancomycin Injection, USP in 0.9% Sodium Chloride
500 mg/100 mL (5 mg/mL) in single dose GALAXY container
750 mg/150 mL (5 mg/mL) in single dose GALAXY container
1 g/200 mL (5 mg/mL) in single dose GALAXY container
Indications and Important Risk Information
Indications
Vancomycin Injection is a glycopeptide antibacterial indicated for the treatment of the following infections in adult and pediatric patients for whom appropriate dosing with this formulation can be achieved:
- Septicemia
- Infective Endocarditis
- Skin and Skin Structure Infections
- Bone Infections
- Lower Respiratory Tract Infections
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Vancomycin Injection and other antibacterial drugs, Vancomycin Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
-
Contraindications: Vancomycin Injection is contraindicated in patients with known hypersensitivity to vancomycin. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
-
Infusion Reactions: Hypotension, including shock and cardiac arrest, wheezing, dyspnea, urticaria or pruritus, muscular and chest pain may occur with rapid Vancomycin Injection administration. The reactions may be more severe in pediatric patients. Rapid intravenous administration may also be associated with “vancomycin infusion reaction”, which manifests as pruritus and erythema that involves the face, neck and upper body. There have been reports that the frequency of infusion-related reactions increases with the concomitant administration of anesthetic agents. Infusion-related adverse reactions are related to both the concentration and rate of administration.
Administer Vancomycin Injection over a period of 60 minutes or greater prior to administration of anesthetic agents when feasible. Stopping the infusion usually results in prompt cessation of these reactions. -
Nephrotoxicity: Vancomycin Injection can result in acute kidney injury (AKI), including acute renal failure. The risk of AKI increases with higher vancomycin serum levels, prolonged exposure, concomitant administration of other nephrotoxic drugs, concomitant administration of piperacillin-tazobactam, volume depletion, pre-existing renal impairment and in critically ill patients and patients with co-morbid conditions that predispose to renal impairment. Monitor serum vancomycin concentrations and renal function in all patients. If AKI occurs, discontinue Vancomycin Injection, or reduce the dose.
-
Ototoxicity: Has occurred in patients receiving Vancomycin Injection. It may be reversible or permanent. Ototoxicity manifests as tinnitus, hearing loss, dizziness or vertigo. The risk is higher in older patients, patients who are receiving higher doses, who have an underlying hearing loss, who are receiving concomitant therapy with another ototoxic agent, or who have underlying renal impairment. Monitor serum vancomycin concentrations and renal function in all patients. Discontinue Vancomycin Injection if ototoxicity occurs. Serial tests of auditory function may be helpful in order to minimize the risk.
-
Severe Dermatologic Reactions: Toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), drug reaction with eosinophilia and systemic symptoms (DRESS), acute generalized exanthematous pustulosis (AGEP), and linear IgA bullous dermatosis (LABD) have been reported. Cutaneous signs or symptoms reported include skin rashes, mucosal lesions, and blisters. Discontinue Vancomycin Injection at the first appearance of signs and symptoms of TEN, SJS, DRESS, AGEP, or LABD.
-
Clostridioides difficile associated diarrhea (CDAD): CDAD has been reported with use of nearly all antibacterial agents, including Vancomycin Injection, and may range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
Hemorrhagic Occlusive Retinal Vasculitis (HORV): HORV, including permanent loss of vision, occurred in patients receiving intracameral or intravitreal administration of vancomycin during or after cataract surgery. The safety and efficacy of vancomycin administered by the intracameral or the intravitreal route have not been established.
-
Neutropenia: Reversible neutropenia has been reported. Patients who will undergo prolonged therapy with vancomycin or those who are receiving concomitant drugs that may cause neutropenia should have periodic monitoring of the leukocyte count. Neutropenia appears to be promptly reversible when vancomycin is discontinued.
-
Phlebitis and Other Administration Site Reactions: Inflammation at the injection site has been reported. Vancomycin is irritating to tissue and must be given by a secure intravenous route of administration. Thrombophlebitis may occur, the frequency and severity of which can be minimized by slow infusion of the drug and by rotation of venous access sites.
-
Adverse Reactions: The most common adverse reactions are anaphylaxis, “vancomycin infusion reaction”, acute kidney injury, hearing loss, neutropenia.
-
Drug Interactions:
-
Anesthetic Agents: Concomitant administration of vancomycin and anesthetic agents has been associated with erythema and histamine-like flushing.
-
Piperacillin/Tazobactam: Increased incidence of acute kidney injury in patients receiving concomitant piperacillin/tazobactam as compared to vancomycin alone. Monitor kidney function in patients.
-
-
Renal Impairment: Dosage adjustment of Vancomycin Injection must be made in patients with impaired renal function. Measure vancomycin serum concentrations to guide intravenous therapy, especially in patients with impaired renal function or fluctuating renal function.
Please see accompanying full Prescribing Information for Vancomycin Injection, USP.
ZOSYN (piperacillin and tazobactam) Injection
2.25 g/50 mL, 3.375 g/50 mL, 4.5 g/ 100 mL
Indications and Important Risk Information
Indications
Zosyn is a combination of piperacillin, a penicillin-class antibacterial and tazobactam, a beta-lactamase inhibitor, indicated for the treatment of:
• Intra-abdominal infections in adult and pediatric patients 2 months of age and older
• Nosocomial pneumonia in adult and pediatric patients 2 months of age and older
• Skin and skin structure infections in adults
• Female pelvic infections in adults
• Community-acquired pneumonia in adults
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Zosyn and other antibacterial drugs, Zosyn should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
Contraindications
Zosyn is contraindicated in patients with a history of allergic reactions to any of the penicillins, cephalosporins, or beta-lactamase inhibitors.
Warnings and Precautions
- Hypersensitivity Adverse Reactions: Serious and occasionally fatal hypersensitivity (anaphylactic/anaphylactoid) reactions (including shock) have been reported in patients receiving therapy with Zosyn. These reactions are more likely to occur in individuals with a history of penicillin, cephalosporin, or carbapenem hypersensitivity or a history of sensitivity to multiple allergens. If an allergic reaction occurs, Zosyn should be discontinued and appropriate therapy instituted.
- Severe Cutaneous Adverse Reactions: Zosyn may cause severe cutaneous adverse reactions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis. If patients develop a skin rash they should be monitored closely and Zosyn discontinued if lesions progress.
- Hemophagocytic Lymphohistiocytosis (HLH): Cases of HLH have been reported in pediatric and adult patients treated with Zosyn. Signs and symptoms of HLH may include fever, rash, lymphadenopathy, hepatosplenomegaly and cytopenia. If HLH is suspected, discontinue Zosyn immediately and institute appropriate management.
- Hematologic Adverse Reactions: Bleeding manifestations have occurred in some patients receiving beta-lactam drugs, including piperacillin. These reactions have sometimes been associated with abnormalities of coagulation tests such as clotting time, platelet aggregation and prothrombin time, and are more likely to occur in patients with renal failure. If bleeding manifestations occur, Zosyn should be discontinued and appropriate therapy instituted. The leukopenia/neutropenia associated with Zosyn administration appears to be reversible and most frequently associated with prolonged administration.
Periodic assessment of hematopoietic function should be performed, especially with prolonged therapy, i.e., ≥ 21 days. - Central Nervous System Adverse Reactions: As with other penicillins, Zosyn may cause neuromuscular excitability or seizures. Patients receiving higher doses, especially patients with renal impairment may be at greater risk for central nervous system adverse reactions. Closely monitor patients with renal impairment or seizure disorders for signs and symptoms of neuromuscular excitability or seizures.
- Nephrotoxicity in Critically Ill Patients: The use of Zosyn was found to be an independent risk factor for renal failure and was associated with delayed recovery of renal function as compared to other beta-lactam antibacterial drugs in critically ill patients. Alternative treatment options should be considered in the critically ill population. If alternative treatment options are inadequate or unavailable, monitor renal function during treatment with Zosyn.
- Clostridioides difficile-associated diarrhea (CDAD): CDAD has been reported with use of nearly all antibacterial agents, including Zosyn, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. - Adverse Reactions: The most common adverse reactions (incidence >5%) are diarrhea, constipation, nausea, headache, and insomnia.
- Renal Impairment: In patients with creatinine clearance ≤ 40 mL/min and dialysis patients (hemodialysis and CAPD), the intravenous dose of Zosyn should be reduced to the degree of renal function impairment.
- Drug Interactions:
- Zosyn administration can significantly reduce tobramycin concentrations in hemodialysis patients. Monitor tobramycin concentrations in these patients.
- Probenecid prolongs the half-lives of piperacillin and tazobactam and should not be co-administered with Zosyn unless the benefit outweighs the risk.
- Co-administration of Zosyn with vancomycin may increase the incidence of acute kidney injury. Monitor kidney function in patients receiving Zosyn and vancomycin.
- Monitor coagulation parameters in patients receiving Zosyn and heparin or oral anticoagulants.
- Zosyn may prolong the neuromuscular blockade of vecuronium and other non-depolarizing neuromuscular blockers. Monitor for adverse reactions related to neuromuscular blockade.
Please see accompanying full Prescribing Information for Zosyn.
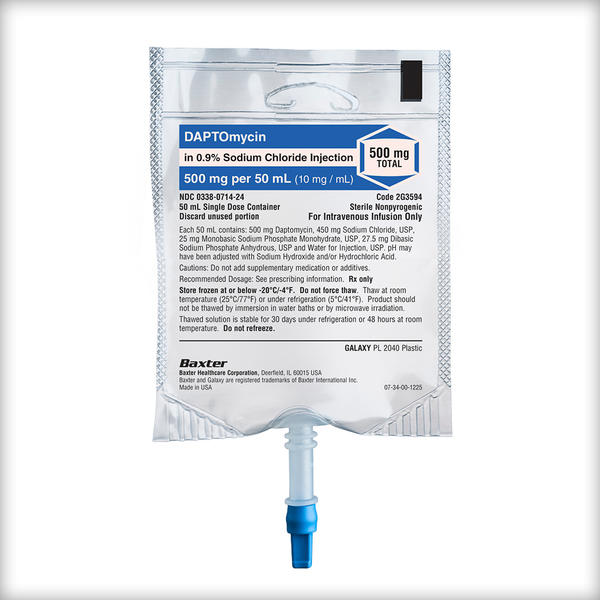
Daptomycin in 0.9% Sodium Chloride Injection
350 mg/50 mL, 500 mg/50 mL, 700 mg/100 mL, 1,000 mg/100 mL
Indications and Important Risk Information
Indications
Daptomycin in Sodium Chloride Injection is a lipopeptide antibacterial indicated for the treatment of:
• Complicated skin and skin structure infections (cSSSI) in adult and pediatric patients (1 to 17 years of age) for whom appropriate dosing can be achieved and,
• Staphylococcus aureus bloodstream infections (bacteremia), in adult patients for whom appropriate dosing can be achieved, including those with right-sided infective endocarditis,
• Staphylococcus aureus bloodstream infections (bacteremia) in pediatric patients (1 to 17 years of age) for whom appropriate dosing can be achieved.
Limitations of Use:
• Daptomycin in Sodium Chloride Injection is not indicated for the treatment of pneumonia.
• Daptomycin in Sodium Chloride Injection is not indicated for the treatment of left-sided infective endocarditis due to S. aureus.
• Daptomycin in Sodium Chloride Injection is not recommended in pediatric patients younger than one year of age due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Daptomycin in Sodium Chloride Injection and other antibacterial drugs, Daptomycin in Sodium Chloride Injection should be used to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Important Risk Information
Contraindications
• Daptomycin in Sodium Chloride Injection is contraindicated in patients with a known hypersensitivity to daptomycin.
Warnings and Precautions
- Anaphylaxis/Hypersensitivity Reactions: Anaphylaxis/hypersensitivity reactions have been reported with the use of antibacterial agents, including daptomycin for injection, and may be life-threatening. If an allergic reaction occurs, discontinue the drug and institute appropriate therapy.
- Myopathy and Rhabdomyolysis: Patients receiving Daptomycin in Sodium Chloride Injection should be monitored for the development of muscle pain or weakness, particularly of the distal extremities. CPK levels should be monitored weekly, and more frequently in patients who received recent, prior, or concomitant therapy with an HMG-CoA reductase inhibitor or in whom elevations in CPK occur during treatment.
In adult patients with renal impairment, both renal function and CPK should be monitored more frequently than once weekly.
Daptomycin in Sodium Chloride Injection should not be dosed more frequently than once a day.
Daptomycin in Sodium Chloride Injection should be discontinued in patients with unexplained signs and symptoms of myopathy in conjunction with CPK elevations to levels >1,000 U/L, and in patients without reported symptoms who have marked elevations in CPK, with levels >2,000 U/L. - Eosinophilic Pneumonia: Has been reported in patients receiving daptomycin for injection. In reported cases, patients developed fever, dyspnea with hypoxic respiratory insufficiency, and diffuse pulmonary infiltrates or organizing pneumonia. In general, patients developed eosinophilic pneumonia 2 to 4 weeks after starting daptomycin for injection and improved when discontinued and steroid therapy was initiated. Recurrence of eosinophilic pneumonia upon re-exposure has been reported. Patients who develop these signs and symptoms should undergo prompt medical evaluation, and Daptomycin in Sodium Chloride Injection should be discontinued immediately.
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS): DRESS has been reported in postmarketing experience. Patients who develop skin rash, fever, peripheral eosinophilia, and systemic organ (for example, hepatic, renal, pulmonary) impairment while receiving Daptomycin in Sodium Chloride Injection should undergo medical evaluation. If DRESS is suspected, discontinue promptly and institute appropriate treatment.
- Tubulointerstitial Nephritis (TIN): TIN has been reported in post-marketing experience. Patients who develop new or worsening renal impairment while receiving Daptomycin in Sodium Chloride Injection should undergo medical evaluation. If TIN is suspected, discontinue promptly and institute appropriate treatment.
- Peripheral Neuropathy: Cases have been reported during post-marketing experience. Therefore, physicians should be alert to signs and symptoms of peripheral neuropathy in patients receiving Daptomycin in Sodium Chloride Injection. Monitor for neuropathy and consider discontinuation.
- Potential Nervous System and/or Muscular System Effects in Pediatric Patients Younger than 12 Months: Avoid use in pediatric patients younger than 12 months due to the risk of potential effects on muscular, neuromuscular, and/or nervous systems (either peripheral and/or central) observed in neonatal dogs with intravenous daptomycin.
- Clostridioides difficile-Associated Diarrhea (CDAD): CDAD has been reported with the use of nearly all systemic antibacterial agents, including daptomycin for injection, and may range in severity from mild diarrhea to fatal colitis. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
- Persisting or Relapsing S. aureus Bacteremia/Endocarditis: Patients should have repeat blood cultures. If a blood culture is positive for S. aureus, minimum inhibitory concentration (MIC) susceptibility testing of the isolate should be performed, and diagnostic evaluation of the patient should be performed to rule out sequestered foci of infection. Appropriate surgical intervention and/or consideration of a change in antibacterial regimen may be required. Failure of treatment may be due to reduced daptomycin susceptibility.
- Decreased efficacy was observed in adult patients with moderate baseline renal impairment: Consider these data when selecting antibacterial therapy for use in adult patients with baseline moderate to severe renal impairment.
- Adverse Reactions:
- Adult cSSSI Patients: The most common adverse reactions that occurred in ≥2% of adult cSSSI patients receiving daptomycin for injection 4 mg/kg were diarrhea, headache, dizziness, rash, abnormal liver function tests, elevated creatine phosphokinase (CPK), urinary tract infections, hypotension, and dyspnea.
- Pediatric cSSSI Patients: The most common adverse reactions that occurred in ≥2% of pediatric patients receiving daptomycin for injection were diarrhea, vomiting, abdominal pain, pruritus, pyrexia, elevated CPK, and headache.
- Adult S. aureus bacteremia/endocarditis Patients: The most common adverse reactions that occurred in ≥5% of S. aureus bacteremia/endocarditis patients receiving daptomycin for injection 6 mg/kg were sepsis, bacteremia, abdominal pain, chest pain, edema, pharyngolaryngeal pain, pruritus, increased sweating, insomnia, elevated CPK, and hypertension.
- Pediatric S. aureus bacteremia Patients: The most common adverse reactions that occurred in ≥5% of pediatric patients receiving daptomycin for injection were vomiting and elevated CPK.
- Drug Interactions:
- HMG-CoA Reductase Inhibitors: Inhibitors of HMG-CoA reductase may cause myopathy. Experience with the coadministration of HMG-CoA reductase inhibitors and daptomycin for injection in patients is limited; therefore, consideration should be given to suspending use of HMG-CoA reductase inhibitors temporarily in patients receiving Daptomycin in Sodium Chloride Injection.
- Drug-Lab Test Interactions: Increased International Normalized Ratio (INR)/Prolonged Prothrombin Time: Clinically relevant plasma concentrations of daptomycin have been observed to cause a significant concentration-dependent false prolongation of prothrombin time (PT) and elevation of International Normalized Ratio (INR) when certain recombinant thromboplastin reagents are utilized for the assay.
Dosage and Administration
• If a dose of Daptomycin in Sodium Chloride Injection is required that does not equal 350 mg, 500 mg, 700 mg or 1,000 mg, this product is not recommended for use and an alternative formulation of daptomycin should be considered.
Please see accompanying full Prescribing Information for Daptomycin in 0.9% Sodium Chloride Injection.
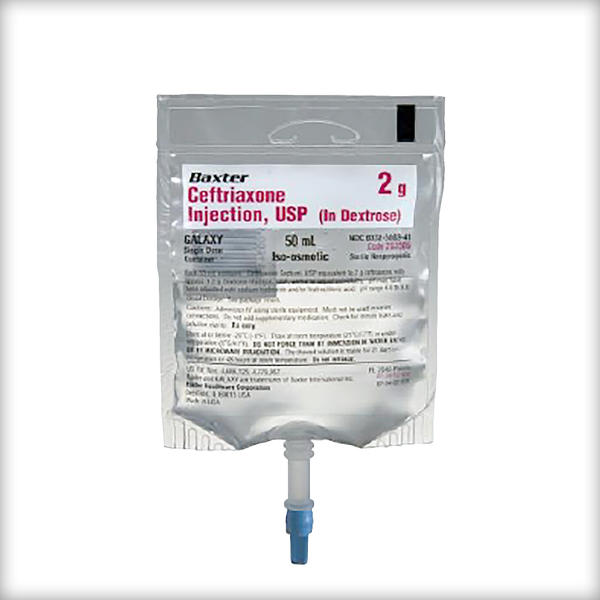
Ceftriaxone Injection, USP (in Dextrose)
1 gram/50 mL
2 gram/50 mL
Indications and Important Risk Information
Indications
Ceftriaxone Injection is indicated for the treatment of the following infections when caused by susceptible organisms: Lower Respiratory Tract Infections, Acute Bacterial Otitis Media, Skin and Skin Structure Infections, Urinary Tract Infections (complicated and uncomplicated), Uncomplicated Gonorrhea (cervical/urethral and rectal), Pelvic Inflammatory Disease, Bacterial Septicemia, Bone and Joint Infections, Intra-Abdominal Infections, Meningitis, and Surgical Prophylaxis.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ceftriaxone Injection and other antibacterial drugs, Ceftriaxone Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
Important Risk Information
• Contraindications: Ceftriaxone Injection is contraindicated in patients with known allergy to the cephalosporin class of antibiotics. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
Neonates (≤28 days): Hyperbilirubinemic neonates, especially prematures, should not be treated with Ceftriaxone Injection.
Ceftriaxone Injection is contraindicated in neonates if they require (or are expected to require) treatment with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone-calcium. Fatalities have been reported.
• Hypersensitivity: Before therapy with Ceftriaxone Injection is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to Cephalosporins, Penicillins or other drugs. This product should be given cautiously to Penicillin-sensitive patients. As with other cephalosporins, anaphylactic reactions with fatal outcome have been reported, even if a patient is not known to be allergic or previously exposed.
• Interaction with Calcium-Containing Products: Do not further dilute ceftriaxone injection with products containing calcium because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same IV administration line. Ceftriaxone must not be administered simultaneously with calcium-containing IV solutions via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially if the infusion lines are thoroughly flushed between infusions.
• Neurological Adverse Reactions: Serious neurological adverse reactions have been reported during postmarketing surveillance, including encephalopathy, seizures, myoclonus, and non-convulsive status epilepticus. Some cases occurred in patients with severe renal impairment. The neurological adverse reactions were reversible and resolved after discontinuation. If neurological adverse reactions occur, discontinue Ceftriaxone Injection. Make dosage adjustments in patients with severe renal impairment.
• Clostridioides difficile associated diarrhea (CDAD): May range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
• Hemolytic Anemia: An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials including ceftriaxone. Severe cases of hemolytic anemia, including fatalities, have been reported during treatment in both adults and children. If a patient develops anemia, stop ceftriaxone until the etiology is determined.
• Gallbladder abnormalities: There have been reports of sonographic abnormalities in the gallbladder of patients treated with ceftriaxone; some of these patients also had symptoms of gallbladder disease. The condition appears to be transient and reversible upon discontinuation of ceftriaxone and institution of conservative management.
• Adverse Reactions: The most common adverse reactions were eosinophilia (6%), thrombocytosis (5.1%), leukopenia (2.1%), diarrhea (2.7%), and elevations of SGOT (3.1%) or SGPT (3.3%).
Please see accompanying full Prescribing Information for Ceftriaxone Injection.
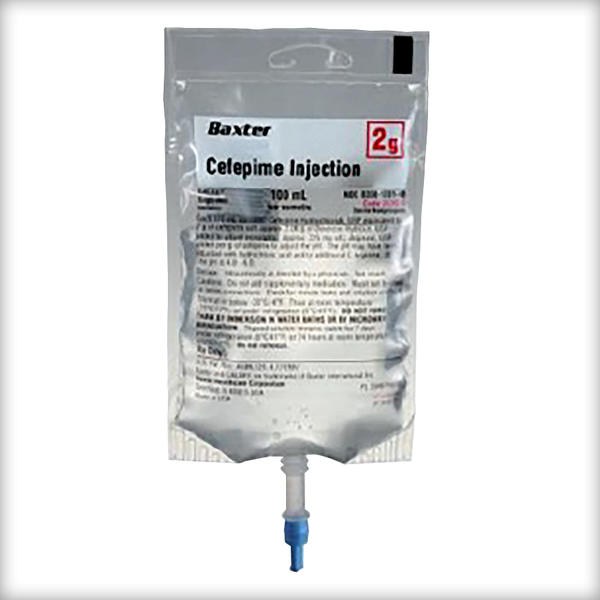
Cefepime Injection
1 gram/50 mL
2 grams/100 mL
Indications and Important Risk Information
Indications
Cefepime Injection is a cephalosporin antibacterial indicated in the treatment of the following infections caused by susceptible isolates of the designated microorganisms: pneumonia; empiric therapy for febrile neutropenic patients; uncomplicated and complicated urinary tract infections; uncomplicated skin and skin structure infections; and complicated intra-abdominal infections (used in combination with metronidazole).
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefepime Injection and other antibacterial drugs, Cefepime Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Important Risk Information
• Contraindications: Prior immediate hypersensitivity reactions to cefepime or the cephalosporin class of antibacterial drugs, penicillins, and other beta-lactam antibacterial drugs. Solutions containing dextrose may be contraindicated in patients with known allergy to corn or corn products.
• Hypersensitivity Reactions: Before therapy with Cefepime Injection, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefepime, cephalosporins, penicillins, or other beta-lactams. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibacterial drugs may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue the drug.
• Neurotoxicity: Serious adverse reactions have been reported including life-threatening or fatal occurrences of the following: encephalopathy, aphasia, myoclonus, seizures, and nonconvulsive status epilepticus. Most cases occurred in patients with renal impairment who did not receive appropriate dosage adjustment. In the majority of cases, symptoms of neurotoxicity were reversible and resolved after discontinuation of cefepime and/or after hemodialysis. If neurotoxicity associated with cefepime therapy occurs, discontinue cefepime.
• Clostridioides difficile associated diarrhea (CDAD): May range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
• Adverse Reactions: The most common adverse reactions (incidence ≥ 1 %) were local reactions, positive Coombs' test, decreased phosphorous, increased ALT and AST, increased PT and PTT and rash. At the highest dose (2 g every 8 hours), incidence of adverse reactions was ≥ 1% for rash, diarrhea, nausea, vomiting, pruritis, fever, and headache.
• Geriatric Use: Serious adverse reactions have occurred in geriatric patients with renal impairment given unadjusted doses of cefepime.
• Drug Interactions:
-
Aminoglycosides: Renal function should be monitored carefully if high doses of aminoglycosides are to be administered with Cefepime Injection because of the increased potential of nephrotoxicity and ototoxicity.
-
Diuretics: Nephrotoxicity has been reported following concomitant administration of other cephalosporins with potent diuretics such as furosemide.
Please see accompanying full Prescribing Information for Cefepime Injection.
Cefazolin in Dextrose Injection, USP
1 gram/50 mL
2 grams/100 mL
3 grams/150 mL
Indications and Important Risk Information
Indications
Cefazolin in Dextrose Injection is a cephalosporin antibacterial indicated for:
-
Treatment of respiratory tract infections in adults and pediatric patients for whom appropriate dosing with this formulation can be achieved.
Limitations of Use: Injectable benzathine penicillin is considered the drug of choice in treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever. -
Treatment of the following infections caused by susceptible isolates of the designated microorganisms in adult and pediatric patients for whom appropriate dosing with this formulation can be achieved: Urinary tract infections; Skin and skin structure infections; Biliary tract infections; Bone and joint infections; Genital infections; Septicemia; Endocarditis.
-
Perioperative prophylaxis in adults and pediatric patients aged 10 – 17 years old for whom appropriate dosing with this formulation can be achieved.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefazolin in Dextrose Injection and other antibacterial drugs, Cefazolin in Dextrose Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Important Risk Information
-
Contraindications: Hypersensitivity to cefazolin or other cephalosporin class antibacterial drugs, penicillins, or other beta-lactams.
-
Hypersensitivity Reactions to Cefazolin, Cephalosporins, Penicillins, or Other Beta-lactams: Serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported in patients receiving beta-lactam antibacterial drugs. Before therapy with Cefazolin in Dextrose Injection, careful inquiry should be made to determine whether the patient has had previous immediate hypersensitivity reactions to cefazolin, cephalosporins, penicillins, or carbapenems. Exercise caution if this product is to be given to penicillin-sensitive patients because cross-hypersensitivity among beta-lactam antibacterial drugs may occur in up to 10% of patients with a history of penicillin allergy. If an allergic reaction occurs, discontinue the drug.
-
Seizures in Patients with Renal Impairment: Seizures may occur particularly in patients with renal impairment when the dosage is not reduced appropriately. Discontinue Cefazolin in Dextrose Injection if seizures occur or make appropriate dosage adjustments in patients with renal impairment. Anticonvulsant therapy should be continued in patients with known seizure disorders.
-
Clostridioides difficile-associated Diarrhea (CDAD): May range in severity from mild diarrhea to fatal colitis. CDAD must be considered in all patients who present with diarrhea following antibiotic use. If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
Prothrombin Activity: Cefazolin in Dextrose Injection may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous vitamin K administered as indicated.
-
Adverse Reactions: Adult and Pediatric Patients: Most common adverse reactions: gastrointestinal (nausea, vomiting, diarrhea), and allergic reactions (anaphylaxis, urticaria, skin rash).
Pediatric Patients with Perioperative Prophylaxis: The most frequently reported adverse reactions (incidence ≥ 5%) were nausea, infusion site pain, and headache. -
Drug Interactions:
-
Probenecid: The renal excretion of cefazolin is inhibited by probenecid. Co-administration of probenecid with Cefazolin in Dextrose Injection is not recommended.
-
Please see accompanying full Prescribing Information for Cefazolin in Dextrose Injection, USP.



![Zosyn [Piperacillin and tazobactam] Injection](/sites/g/files/ebysai2186/files/styles/profile_card/public/2024-07/299.32%20Frozen%20Portfolio%20Landing%20Page_Rv6.27.jpg?h=68256770&itok=v9B6pk4e)